HackTwat
MuscleChemistry Registered Member
[h=3]Anabolic-androgenic Steroids and TAFLD
Abstract and Introduction[/h][h=4]Abstract[/h]Background: Industrial toxin and drugs have been associated with non-alcoholic fatty liver disease (NAFLD); in these cases, the disease has been termed toxicant-associated steatohepatitis (TASH).
Aim: This study hypothesizes that the use of anabolic-androgenic steroids (AAS) could also be a risk factor to TASH or better toxicant-associated fatty liver disease (TAFLD) development.
Methodology: Case–control study including 180 non-competitive recreational male bodybuilders from August/2007 to March/2009. Ninety-five had a history of intramuscular AAS use (cases; G1) and 85 were non-users (controls; G2). They underwent a clinical evaluation and abdominal ultrasound, and their blood levels of aminotransferases, creatine phosphokinase (CPK), lipids, glucose and insulin were measured. TAFLD criteria: history of AAS use >2 years; presence of hepatic steatosis on ultrasound and/or aminotransferase alterations with normal CPK levels; exclusion of ethanol intake ≥20 g/day or use of other drugs; and exclusion of obesity, dyslipidaemia, diabetes and other liver diseases. Homeostasis model assessment for insulin resistance ≥3 was considered insulin resistant. Independent t-test, odds ratio (OR) and 95% confidence intervals (95% CI) were calculated.
Results: All cases were asymptomatic. Clinical and laboratorial data were similar in G1 and G2 ( P>0.05). TAFLD criteria were observed in 12.6% of the G1 cases and 2.4% of controls had criteria compliant with non-alcoholic fatty liver related to metabolic conditions. OR was 6.0 (95% CI: 1.3–27.6).
Conclusions: These results suggest that AAS could be a possible new risk factor for TAFLD. In this type of fatty liver disease, the individuals had a low body fat mass and they did not present insulin resistance.
[h=4]Introduction[/h]The use of performance-enhancing drugs has grown in recent years.[SUP] [/SUP]Athletes and non-athletes have been using exogenous anabolic-androgenic steroids (AAS) to increase lean body mass to achieve higher levels of sports performance or for aesthetic purposes.[SUP] [/SUP]In the US, approximately 3 million of individuals use non-therapeutic AAS supra-physiological doses and 60% of this population is inclusive of non-competitive recreational bodybuilders.[SUP] [/SUP]In Brazil, the prevalence of AAS users is estimated in 0.9%.[SUP] [/SUP]
Most of the protocols involving AAS users consist of 8 weeks and doses frequently reach more than a 100-fold the physiological concentration. In addition, doses of AAS are composed by a cocktail of many oral and intramuscular testosterone-derived compounds.[SUP] [/SUP]
Non-alcoholic fatty liver disease (NAFLD) is the most common type of chronic liver injury in many countries and its spectrum is inclusive of steatosis, non-alcoholic steatohepatitis, cirrhosis and hepatocellular carcinoma.[SUP] [/SUP]It has been related to obesity, diabetes and dyslipidaemia, which represent a cluster of cardiovascular risk factors, associated with insulin resistance (IR) and metabolic syndrome.[SUP] [/SUP]However, NAFLD has also been associated with drug use, chemical exposure and toxin.
Cases of NAFLD associated with toxic substances have been denominated toxicant-associated steatohepatitis,[SUP] [/SUP]and the present study hypothesizes that AAS use could also be a risk factor for toxicant-associated fatty liver disease (TAFLD) development.
[h=3]Material and Methods[/h][h=4]Study Design and Population Selection[/h]A case–control study included 180 non-competitive recreational male bodybuilders engaged in specific regular physical training from August 2007 to March 2009. Ninety-five had a history of intramuscular AAS use for >2 years (cases; G1) and 85 were non-users (controls; G2). AAS were not prescribed, nor was their use supported. Participants did not receive compensation, but individual test results were discussed with the subjects and follow-up of abnormal results was encouraged.
The study was approved by the Ethics Committee for Human Research from MCO – Programa de Pós-Graduação em Medicina e Saúde (PPGMS) – Universidade Federal da Bahia (UFBA), Brazil. It was conducted according to the principles outlined in the Declaration of Helsinki. All participants provided written informed consent before enrolment.
[h=4]Clinical Evaluation[/h]The parameters studied included age, anthropometric measures, dose and time of AAS use, risk factors (obesity, dyslipidaemia and diabetes), alcohol intake and other illicit drugs use. Laboratory evaluation included HBsAg, hepatitis C antibody (anti-HCV), alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyl transpeptidase (GGT), creatine phosphokinase (CPK), total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol, triglycerides, fasting plasma glucose and insulin. Abdominal ultrasound (AUS) exam was performed in all cases and controls. Obesity was defined as a body mass index (BMI) ≥30 kg/m [SUP]2[/SUP] or a fat mass ≥20%. The diagnosis of diabetes was based on the criteria stated by the American Diabetes Association.
The criteria for TAFLD included a history of ethanol intake ≤20 g/day; a history of AAS use for >2 years; hepatic steatosis on AUS; and/or aminotransferases elevated levels with normal CPK (24–190 U/L). Obesity, dyslipidaemia, diabetes and the use of any other drugs and other liver diseases (B and C hepatitis virus, haemochromatosis and autoimmune hepatitis) were also excluded.
[h=4]Measurements[/h]Height was recorded using a scientific stadiometer (Sanny, São Bernardo do Campo, SP, Brazil) and body weight was measured on a PL-180 digital scale (Filizola, São Paulo, SP, Brazil). Waist and hip circumferences were evaluated with the help of a metallic ribbon (Sanny) and skinfold thickness at the chest, abdominal and mid-thigh were measured using Lange® skinfold calipers (Beta Technology Inc., Santa Cruz, CA, USA). An experienced evaluator using standard protocols performed all anthropometric measurements. Fat content was estimated according to the method proposed by Jackson and Pollock. [SUP] [/SUP]BMIs were calculated based on the aforementioned anthropometric measurements in accordance with the World Health Organization.
Information on drug use, including AAS, and alcohol intake behaviour was obtained through a face-to-face interview conducted by a practitioner. Information on the strength training regimen was also obtained through an interview, and the intensity has often being assigned based on one maximum repetition (1 RM) for exercises performed in a training session.[SUP] [/SUP]
Insulin resistance (IR) was calculated using the homeostasis model assessment for IR (HOMA-IR). HOMA-IR ≥3 was considered to indicate IR.
Abdominal ultrasound images were obtained by an experienced sonographer and liver steatosis was classified as focal, diffuse (mild, moderate, severe) and/or non-specific.
[h=4]Statistical Analysis[/h]Data were processed and analysed using spss (SPSS Inc., Chicago, IL, USA, Release 16.0.2, 2008) and prism (GraphPad Inc., San Diego, CA, USA, Release 5.01) softwares. Initially, descriptive statistics were applied to the Kolmogorov–Smirnov test and to Bartlett's criteria. Continuous variables were summarized with means and standard deviations while categorical variables were presented in frequencies and percentages. Continuous data were analysed using Student's t-test for independent samples. Pearson's correlation coefficients were calculated to check the degree of association between the variables studied. Fisher's exact test was used for comparison of frequency data. Proportions of non-competitive recreational bodybuilders with TAFLD diagnosis and non-alcoholic fatty liver criteria between the G1 and G2, respectively, were calculated to estimate the odds ratio (OR). All statistical methods were two-tailed, P values were calculated and significance level was set to <0.05.
[h=3]Results[/h]Non-competitive recreational bodybuilders AAS users with a TAFLD diagnosis (G1) were younger and had a lower fat mass than non-AAS users.
In G1, the use of AAS occurs in defined periods, often separated by periods of non-use (cycles). Eighty-seven participants reported using two or more intramuscular AAS substances and the others reported using a combination of intramuscular and oral substances. The length of self-reported AAS use ranged from 2 to 10 years (mean of 4 years). AAS doses of testosterone or its equivalent in the last cycle ranged from 200 to 5200 mg, with a mean of 1200 mg. The AAS intramuscular injections reported were testosterone, nandrolone, boldenone, methenolone and stanozolol and the oral preparations combined in the cycle were methandrostenolone, oxymetholone and oxandrolone.
Nearly 42% ( n=40) in G1 and 62% ( n=53) in G2 were single or lived alone. Achievement of secondary-level education or higher was reported for >82% ( n=78) in G1 and 91% ( n=78) in G2. The means of age, the total body mass and all other anthropometric measures were similar in both groups. All recreational bodybuilders screened presented negative tests for hepatitis B and C viruses. Blood analysis also showed similar means of CPK enzyme levels, insulin, HOMA-IR, blood lipid profile and GGT among groups. Nevertheless, the AST and ALT means were significantly higher among the cases than that in the controls ( P<0.01), and the mean fasting plasma glucose by G1 was significantly lower compared with G2 ( Table 1).
According to volunteers' reports, the strength training frequency ranged from 5 to 7 days per week in G1 (AAS users) and 4–7 days per week in G2 (non-AAS users). The self-reported intensity for the strength training was 78 and 75% of 1 RM, respectively, in G1 and G2 ( Table 2).
Obesity according to BMI was found in four cases and 10 controls; however, only two controls presented these criteria using a fat mass cut-off value. Only one case presented diabetes mellitus. Dyslipidaemia was found in 19% ( n=18) and 18% ( n=15) between G1 and G2 respectively. It was associated with lower levels of HDL in 89% of G1 and in 67% of G2.
Elevated CPK enzyme levels were found in approximately 55% ( n=52) in G1 and in 45% ( n=38) of G2 ( P=0.23). Use of other drugs was reported by 26 cases (27%) and 18 controls (21%) ( P=0.39). A history of ethanol consumption for >20 g/day was reported by 29 cases (31%) and by 16 controls (19%) ( P=0.09). Elevated liver enzymes were observed in 33% ( n=31) and 7% ( n=6) between G1 and G2 ( P<0.01). Low, but significant, positive correlations were found among liver enzymes (ALT and AST) and CPK concentrations in G2, and only between ALT and CPK in G1 (Fig. 1).
 (Enlarge Image)
(Enlarge Image)
Figure 1.
Correlation between hepatic enzymes and CPK levels of non-competitive recreational bodybuilders AAS users (cases; G1) and non-AAS users (controls; G2). AAS, anabolic-androgenic steroids; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CPK, creatine phosphokinase.
Diagnosis criteria for TAFLD were observed in 12.6% ( n=12) among G1 or AAS users. In G2 (controls), one individual presented elevated ALT (64 U/L) and another AST (68 U/L), both with normal CPK levels (176.5±3.5 U/L) and normal AUS images. They represent 2.4% of individuals with non-alcoholic fatty liver criteria in the control group (G2). Based on these proportions, OR for TAFLD development was 6.0 (95% confidence interval, 1.3–27.6).
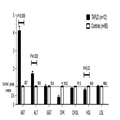
G1 presents AST mean serum levels four times greater than those in the controls and ALT mean serum levels two times higher than that of the same group (Fig. 2). In addition, the mean HDL-C was lower in the TAFLD group. Hepatic enzyme GGT presents similar means between cases and controls; the same results were obtained for other blood clinical markers ( Table 3).
 (Enlarge Image)
(Enlarge Image)
Figure 2.
Enzyme and serum lipid levels' comparison between non-competitive recreational bodybuilders with a diagnosis of toxicant-associated fatty liver disease (TAFLD) and controls. The results from the control group were considered reference values for comparison. ALT, alanine aminotransferase; AST, aspartate aminotransferase; CHOL, total cholesterol; CPK, creatine phosphokinase; GGT, glutamyl transpeptidase; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
All the controls had normal liver images. Twelve AAS users (12.6%) had hepatomegaly alone, and 13 (13.7%) had liver steatosis on AUS. Ten of them had mild diffuse steatosis; two had moderate diffuse steatosis; and one had focal steatosis. Six of these cases also showed hepatomegaly.
[h=3]Discussion[/h]The studies that addressed AAS abuse and liver injury have shown aminotransferase alterations, and hepatic neoplasm depending on the time and the dosage used.[SUP] [/SUP]The relationship between fatty liver and long-term exogenous AAS use is unknown. In preliminary data,[SUP] [/SUP]we reported cases of NAFLD in asymptomatic non-competitive bodybuilders who have been using exogenous AAS to increase lean body mass.
This case–control study confirms the previous results. It shows a high OR of liver injury related to AAS use and suggests the denomination of TAFLD for this condition.
Despite the increase of the use of the AAS in the last few years,[SUP] [/SUP]to date, no other research involving human subjects has been conducted to evaluate the risk of fatty liver disease development among AAS users. AAS therapy is associated with various adverse effects that are generally dose related; therefore, illicit use of the high doses taken by sportsmen carries substantial risks for health.[SUP] [/SUP]A major side effect of AAS therapy is hepatotoxicity, including elevated blood levels of liver enzymes, cholestatic jaundice, peliosis and several neoplasia.[SUP] [/SUP]Studies in liver of rats subjected to short- and long-term AAS treatment have shown steatosis, inflammatory or degenerative lesions in hepatocytes.[SUP] [/SUP]
In parallel to the rise in obesity is also the search for fast body-shaping strategies. Most of these strategies have been linked to physical and physiological damages, which are considered a health problem in some population groups. In this sense, school programmes were designed to prevent this behaviour among teenagers.
The intramuscular use of AAS has been one of the most frequent strategies to speed up increased muscle mass for sports performance or aesthetic purposes, and these drugs have been associated with cardiovascular and other injuries.[SUP] [/SUP]Few studies have shown the association between AAS user and fatty liver disease;[SUP] [/SUP]however, several drugs and toxins have been related to fatty liver and the relationship between AAS and this liver condition needs to be established.
These cases and controls were non-competitive recreational bodybuilders[SUP] [/SUP]and presented normal body fat mass for age and sex. In addition, anthropometric measures and the plasma lipid concentration were similar to those in other studies conducted with AAS users.[SUP] [/SUP]Some individuals reported the use of AAS more than a 100-fold the physiological concentration. The mean of the AAS dose reported in the last month of use (1200 mg) was similar to the doses given to healthy males to evaluate the effects of AAS on muscle mass and muscle protein synthesis.[SUP] [/SUP]However, it was lower than the doses given to healthy untrained men to evaluate AAS effects on the lean body mass and muscle size.
Most AAS, when used exogenously, inhibit the normal process of steroid biosynthesis.[SUP] [/SUP]This means that the first step in steroidal hormone production is hindered and that cholesterol is not converted into pregnenolone, the product of the initial cholesterol side chain cleavage reaction. This can result in cholesterol storage and possibly the creation of a proper environment for fatty liver accumulation.
Charlton et al.[SUP] [/SUP]described an association between low circulating levels of dehydroepiandrosterone (DHEA) and NAFLD severity. Although we have not evaluated the serum concentration of DHEA, lower levels of this steroid hormone were shown among non-therapeutic testosterone users in comparison with non-users.[SUP] [/SUP]In addition, pregnenolone is directed into different synthetic pathways, leading to the formation of various steroids including DHEA, and in AAS exogenous users, this chain reaction is probably compromised.
The participants (cases and controls) did not present IR. They were also lean (BMI and body fat content within normal range values) and younger and were engaged in regular physical training. In this sense, they comprise an unusual population to present fat accumulation in the liver (NAFLD). These conditions are probably the reason for the very low prevalence of steatosis found in the both groups, less than that in the general population.
Bacon et al.[SUP] [/SUP]demonstrated NAFLD in lean patients with normal fasting glucose and glucose tolerance, without increased plasma lipids. Nevertheless, NAFLD in these subjects is normally associated with decreased insulin sensitivity.[SUP] [/SUP]Fasting plasma glucose was significantly higher in the control group and this result deserves further investigation. It is noteworthy that in healthy adult men, testosterone was negatively correlated with fasting plasma glucose and glucose measured 2 h after 75 g glucose load.[SUP] [/SUP]
Analyses of ALT and AST have shown a significantly higher level in individuals with TALFD than that in the control group. These findings are similar to those of others,[SUP] [/SUP]who have demonstrated evidence for enhanced blood aminotransferase activities after AAS use in bodybuilders.
A discussion of the relationship of high levels of ALT, AST and physical training-induced muscle disruption is important. However, in agreement with Pagonis et al.,[SUP] [/SUP]the CPK values, a specific muscle enzyme used to estimate exercise-induced skeletal muscle damage, were not different between cases of TAFLD and controls in this sample. Furthermore, no significant correlation was observed between AST and CPK levels or ALT and CPK. These results may suggest that the observed AST and ALT increase in our sample may represent toxic liver injury associated with AAS use. In agreement with these results, the OR of TAFLD cases was six times higher than that of the control group.
This study did not aim at investigating the pathogenesis of TAFLD; however, when the risk factors to this disease are drugs that induce hepatotoxicity, probably the reactive oxygen species (ROS) are involved. The hypothesis of enhanced ROS production leading to TAFLD has also been supported by the findings of Gragera et al.,[SUP] [/SUP]who showed an increase of mitochondrial swelling and electron-lucent matrix, a known feature of ROS oxidative damage, induced by AAS in the liver of trained rats.
In conclusion, the results suggest that AAS could be a possible new risk factor for TAFLD. In this type of fatty liver disease, the individuals had a low body fat mass and they did not present IR.
Abstract and Introduction[/h][h=4]Abstract[/h]Background: Industrial toxin and drugs have been associated with non-alcoholic fatty liver disease (NAFLD); in these cases, the disease has been termed toxicant-associated steatohepatitis (TASH).
Aim: This study hypothesizes that the use of anabolic-androgenic steroids (AAS) could also be a risk factor to TASH or better toxicant-associated fatty liver disease (TAFLD) development.
Methodology: Case–control study including 180 non-competitive recreational male bodybuilders from August/2007 to March/2009. Ninety-five had a history of intramuscular AAS use (cases; G1) and 85 were non-users (controls; G2). They underwent a clinical evaluation and abdominal ultrasound, and their blood levels of aminotransferases, creatine phosphokinase (CPK), lipids, glucose and insulin were measured. TAFLD criteria: history of AAS use >2 years; presence of hepatic steatosis on ultrasound and/or aminotransferase alterations with normal CPK levels; exclusion of ethanol intake ≥20 g/day or use of other drugs; and exclusion of obesity, dyslipidaemia, diabetes and other liver diseases. Homeostasis model assessment for insulin resistance ≥3 was considered insulin resistant. Independent t-test, odds ratio (OR) and 95% confidence intervals (95% CI) were calculated.
Results: All cases were asymptomatic. Clinical and laboratorial data were similar in G1 and G2 ( P>0.05). TAFLD criteria were observed in 12.6% of the G1 cases and 2.4% of controls had criteria compliant with non-alcoholic fatty liver related to metabolic conditions. OR was 6.0 (95% CI: 1.3–27.6).
Conclusions: These results suggest that AAS could be a possible new risk factor for TAFLD. In this type of fatty liver disease, the individuals had a low body fat mass and they did not present insulin resistance.
[h=4]Introduction[/h]The use of performance-enhancing drugs has grown in recent years.[SUP] [/SUP]Athletes and non-athletes have been using exogenous anabolic-androgenic steroids (AAS) to increase lean body mass to achieve higher levels of sports performance or for aesthetic purposes.[SUP] [/SUP]In the US, approximately 3 million of individuals use non-therapeutic AAS supra-physiological doses and 60% of this population is inclusive of non-competitive recreational bodybuilders.[SUP] [/SUP]In Brazil, the prevalence of AAS users is estimated in 0.9%.[SUP] [/SUP]
Most of the protocols involving AAS users consist of 8 weeks and doses frequently reach more than a 100-fold the physiological concentration. In addition, doses of AAS are composed by a cocktail of many oral and intramuscular testosterone-derived compounds.[SUP] [/SUP]
Non-alcoholic fatty liver disease (NAFLD) is the most common type of chronic liver injury in many countries and its spectrum is inclusive of steatosis, non-alcoholic steatohepatitis, cirrhosis and hepatocellular carcinoma.[SUP] [/SUP]It has been related to obesity, diabetes and dyslipidaemia, which represent a cluster of cardiovascular risk factors, associated with insulin resistance (IR) and metabolic syndrome.[SUP] [/SUP]However, NAFLD has also been associated with drug use, chemical exposure and toxin.
Cases of NAFLD associated with toxic substances have been denominated toxicant-associated steatohepatitis,[SUP] [/SUP]and the present study hypothesizes that AAS use could also be a risk factor for toxicant-associated fatty liver disease (TAFLD) development.
[h=3]Material and Methods[/h][h=4]Study Design and Population Selection[/h]A case–control study included 180 non-competitive recreational male bodybuilders engaged in specific regular physical training from August 2007 to March 2009. Ninety-five had a history of intramuscular AAS use for >2 years (cases; G1) and 85 were non-users (controls; G2). AAS were not prescribed, nor was their use supported. Participants did not receive compensation, but individual test results were discussed with the subjects and follow-up of abnormal results was encouraged.
The study was approved by the Ethics Committee for Human Research from MCO – Programa de Pós-Graduação em Medicina e Saúde (PPGMS) – Universidade Federal da Bahia (UFBA), Brazil. It was conducted according to the principles outlined in the Declaration of Helsinki. All participants provided written informed consent before enrolment.
[h=4]Clinical Evaluation[/h]The parameters studied included age, anthropometric measures, dose and time of AAS use, risk factors (obesity, dyslipidaemia and diabetes), alcohol intake and other illicit drugs use. Laboratory evaluation included HBsAg, hepatitis C antibody (anti-HCV), alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyl transpeptidase (GGT), creatine phosphokinase (CPK), total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol, triglycerides, fasting plasma glucose and insulin. Abdominal ultrasound (AUS) exam was performed in all cases and controls. Obesity was defined as a body mass index (BMI) ≥30 kg/m [SUP]2[/SUP] or a fat mass ≥20%. The diagnosis of diabetes was based on the criteria stated by the American Diabetes Association.
The criteria for TAFLD included a history of ethanol intake ≤20 g/day; a history of AAS use for >2 years; hepatic steatosis on AUS; and/or aminotransferases elevated levels with normal CPK (24–190 U/L). Obesity, dyslipidaemia, diabetes and the use of any other drugs and other liver diseases (B and C hepatitis virus, haemochromatosis and autoimmune hepatitis) were also excluded.
[h=4]Measurements[/h]Height was recorded using a scientific stadiometer (Sanny, São Bernardo do Campo, SP, Brazil) and body weight was measured on a PL-180 digital scale (Filizola, São Paulo, SP, Brazil). Waist and hip circumferences were evaluated with the help of a metallic ribbon (Sanny) and skinfold thickness at the chest, abdominal and mid-thigh were measured using Lange® skinfold calipers (Beta Technology Inc., Santa Cruz, CA, USA). An experienced evaluator using standard protocols performed all anthropometric measurements. Fat content was estimated according to the method proposed by Jackson and Pollock. [SUP] [/SUP]BMIs were calculated based on the aforementioned anthropometric measurements in accordance with the World Health Organization.
Information on drug use, including AAS, and alcohol intake behaviour was obtained through a face-to-face interview conducted by a practitioner. Information on the strength training regimen was also obtained through an interview, and the intensity has often being assigned based on one maximum repetition (1 RM) for exercises performed in a training session.[SUP] [/SUP]
Insulin resistance (IR) was calculated using the homeostasis model assessment for IR (HOMA-IR). HOMA-IR ≥3 was considered to indicate IR.
Abdominal ultrasound images were obtained by an experienced sonographer and liver steatosis was classified as focal, diffuse (mild, moderate, severe) and/or non-specific.
[h=4]Statistical Analysis[/h]Data were processed and analysed using spss (SPSS Inc., Chicago, IL, USA, Release 16.0.2, 2008) and prism (GraphPad Inc., San Diego, CA, USA, Release 5.01) softwares. Initially, descriptive statistics were applied to the Kolmogorov–Smirnov test and to Bartlett's criteria. Continuous variables were summarized with means and standard deviations while categorical variables were presented in frequencies and percentages. Continuous data were analysed using Student's t-test for independent samples. Pearson's correlation coefficients were calculated to check the degree of association between the variables studied. Fisher's exact test was used for comparison of frequency data. Proportions of non-competitive recreational bodybuilders with TAFLD diagnosis and non-alcoholic fatty liver criteria between the G1 and G2, respectively, were calculated to estimate the odds ratio (OR). All statistical methods were two-tailed, P values were calculated and significance level was set to <0.05.
[h=3]Results[/h]Non-competitive recreational bodybuilders AAS users with a TAFLD diagnosis (G1) were younger and had a lower fat mass than non-AAS users.
In G1, the use of AAS occurs in defined periods, often separated by periods of non-use (cycles). Eighty-seven participants reported using two or more intramuscular AAS substances and the others reported using a combination of intramuscular and oral substances. The length of self-reported AAS use ranged from 2 to 10 years (mean of 4 years). AAS doses of testosterone or its equivalent in the last cycle ranged from 200 to 5200 mg, with a mean of 1200 mg. The AAS intramuscular injections reported were testosterone, nandrolone, boldenone, methenolone and stanozolol and the oral preparations combined in the cycle were methandrostenolone, oxymetholone and oxandrolone.
Nearly 42% ( n=40) in G1 and 62% ( n=53) in G2 were single or lived alone. Achievement of secondary-level education or higher was reported for >82% ( n=78) in G1 and 91% ( n=78) in G2. The means of age, the total body mass and all other anthropometric measures were similar in both groups. All recreational bodybuilders screened presented negative tests for hepatitis B and C viruses. Blood analysis also showed similar means of CPK enzyme levels, insulin, HOMA-IR, blood lipid profile and GGT among groups. Nevertheless, the AST and ALT means were significantly higher among the cases than that in the controls ( P<0.01), and the mean fasting plasma glucose by G1 was significantly lower compared with G2 ( Table 1).
According to volunteers' reports, the strength training frequency ranged from 5 to 7 days per week in G1 (AAS users) and 4–7 days per week in G2 (non-AAS users). The self-reported intensity for the strength training was 78 and 75% of 1 RM, respectively, in G1 and G2 ( Table 2).
Obesity according to BMI was found in four cases and 10 controls; however, only two controls presented these criteria using a fat mass cut-off value. Only one case presented diabetes mellitus. Dyslipidaemia was found in 19% ( n=18) and 18% ( n=15) between G1 and G2 respectively. It was associated with lower levels of HDL in 89% of G1 and in 67% of G2.
Elevated CPK enzyme levels were found in approximately 55% ( n=52) in G1 and in 45% ( n=38) of G2 ( P=0.23). Use of other drugs was reported by 26 cases (27%) and 18 controls (21%) ( P=0.39). A history of ethanol consumption for >20 g/day was reported by 29 cases (31%) and by 16 controls (19%) ( P=0.09). Elevated liver enzymes were observed in 33% ( n=31) and 7% ( n=6) between G1 and G2 ( P<0.01). Low, but significant, positive correlations were found among liver enzymes (ALT and AST) and CPK concentrations in G2, and only between ALT and CPK in G1 (Fig. 1).
 (Enlarge Image)
(Enlarge Image) Figure 1.
Correlation between hepatic enzymes and CPK levels of non-competitive recreational bodybuilders AAS users (cases; G1) and non-AAS users (controls; G2). AAS, anabolic-androgenic steroids; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CPK, creatine phosphokinase.
Diagnosis criteria for TAFLD were observed in 12.6% ( n=12) among G1 or AAS users. In G2 (controls), one individual presented elevated ALT (64 U/L) and another AST (68 U/L), both with normal CPK levels (176.5±3.5 U/L) and normal AUS images. They represent 2.4% of individuals with non-alcoholic fatty liver criteria in the control group (G2). Based on these proportions, OR for TAFLD development was 6.0 (95% confidence interval, 1.3–27.6).
G1 presents AST mean serum levels four times greater than those in the controls and ALT mean serum levels two times higher than that of the same group (Fig. 2). In addition, the mean HDL-C was lower in the TAFLD group. Hepatic enzyme GGT presents similar means between cases and controls; the same results were obtained for other blood clinical markers ( Table 3).
 (Enlarge Image)
(Enlarge Image) Figure 2.
Enzyme and serum lipid levels' comparison between non-competitive recreational bodybuilders with a diagnosis of toxicant-associated fatty liver disease (TAFLD) and controls. The results from the control group were considered reference values for comparison. ALT, alanine aminotransferase; AST, aspartate aminotransferase; CHOL, total cholesterol; CPK, creatine phosphokinase; GGT, glutamyl transpeptidase; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
All the controls had normal liver images. Twelve AAS users (12.6%) had hepatomegaly alone, and 13 (13.7%) had liver steatosis on AUS. Ten of them had mild diffuse steatosis; two had moderate diffuse steatosis; and one had focal steatosis. Six of these cases also showed hepatomegaly.
[h=3]Discussion[/h]The studies that addressed AAS abuse and liver injury have shown aminotransferase alterations, and hepatic neoplasm depending on the time and the dosage used.[SUP] [/SUP]The relationship between fatty liver and long-term exogenous AAS use is unknown. In preliminary data,[SUP] [/SUP]we reported cases of NAFLD in asymptomatic non-competitive bodybuilders who have been using exogenous AAS to increase lean body mass.
This case–control study confirms the previous results. It shows a high OR of liver injury related to AAS use and suggests the denomination of TAFLD for this condition.
Despite the increase of the use of the AAS in the last few years,[SUP] [/SUP]to date, no other research involving human subjects has been conducted to evaluate the risk of fatty liver disease development among AAS users. AAS therapy is associated with various adverse effects that are generally dose related; therefore, illicit use of the high doses taken by sportsmen carries substantial risks for health.[SUP] [/SUP]A major side effect of AAS therapy is hepatotoxicity, including elevated blood levels of liver enzymes, cholestatic jaundice, peliosis and several neoplasia.[SUP] [/SUP]Studies in liver of rats subjected to short- and long-term AAS treatment have shown steatosis, inflammatory or degenerative lesions in hepatocytes.[SUP] [/SUP]
In parallel to the rise in obesity is also the search for fast body-shaping strategies. Most of these strategies have been linked to physical and physiological damages, which are considered a health problem in some population groups. In this sense, school programmes were designed to prevent this behaviour among teenagers.
The intramuscular use of AAS has been one of the most frequent strategies to speed up increased muscle mass for sports performance or aesthetic purposes, and these drugs have been associated with cardiovascular and other injuries.[SUP] [/SUP]Few studies have shown the association between AAS user and fatty liver disease;[SUP] [/SUP]however, several drugs and toxins have been related to fatty liver and the relationship between AAS and this liver condition needs to be established.
These cases and controls were non-competitive recreational bodybuilders[SUP] [/SUP]and presented normal body fat mass for age and sex. In addition, anthropometric measures and the plasma lipid concentration were similar to those in other studies conducted with AAS users.[SUP] [/SUP]Some individuals reported the use of AAS more than a 100-fold the physiological concentration. The mean of the AAS dose reported in the last month of use (1200 mg) was similar to the doses given to healthy males to evaluate the effects of AAS on muscle mass and muscle protein synthesis.[SUP] [/SUP]However, it was lower than the doses given to healthy untrained men to evaluate AAS effects on the lean body mass and muscle size.
Most AAS, when used exogenously, inhibit the normal process of steroid biosynthesis.[SUP] [/SUP]This means that the first step in steroidal hormone production is hindered and that cholesterol is not converted into pregnenolone, the product of the initial cholesterol side chain cleavage reaction. This can result in cholesterol storage and possibly the creation of a proper environment for fatty liver accumulation.
Charlton et al.[SUP] [/SUP]described an association between low circulating levels of dehydroepiandrosterone (DHEA) and NAFLD severity. Although we have not evaluated the serum concentration of DHEA, lower levels of this steroid hormone were shown among non-therapeutic testosterone users in comparison with non-users.[SUP] [/SUP]In addition, pregnenolone is directed into different synthetic pathways, leading to the formation of various steroids including DHEA, and in AAS exogenous users, this chain reaction is probably compromised.
The participants (cases and controls) did not present IR. They were also lean (BMI and body fat content within normal range values) and younger and were engaged in regular physical training. In this sense, they comprise an unusual population to present fat accumulation in the liver (NAFLD). These conditions are probably the reason for the very low prevalence of steatosis found in the both groups, less than that in the general population.
Bacon et al.[SUP] [/SUP]demonstrated NAFLD in lean patients with normal fasting glucose and glucose tolerance, without increased plasma lipids. Nevertheless, NAFLD in these subjects is normally associated with decreased insulin sensitivity.[SUP] [/SUP]Fasting plasma glucose was significantly higher in the control group and this result deserves further investigation. It is noteworthy that in healthy adult men, testosterone was negatively correlated with fasting plasma glucose and glucose measured 2 h after 75 g glucose load.[SUP] [/SUP]
Analyses of ALT and AST have shown a significantly higher level in individuals with TALFD than that in the control group. These findings are similar to those of others,[SUP] [/SUP]who have demonstrated evidence for enhanced blood aminotransferase activities after AAS use in bodybuilders.
A discussion of the relationship of high levels of ALT, AST and physical training-induced muscle disruption is important. However, in agreement with Pagonis et al.,[SUP] [/SUP]the CPK values, a specific muscle enzyme used to estimate exercise-induced skeletal muscle damage, were not different between cases of TAFLD and controls in this sample. Furthermore, no significant correlation was observed between AST and CPK levels or ALT and CPK. These results may suggest that the observed AST and ALT increase in our sample may represent toxic liver injury associated with AAS use. In agreement with these results, the OR of TAFLD cases was six times higher than that of the control group.
This study did not aim at investigating the pathogenesis of TAFLD; however, when the risk factors to this disease are drugs that induce hepatotoxicity, probably the reactive oxygen species (ROS) are involved. The hypothesis of enhanced ROS production leading to TAFLD has also been supported by the findings of Gragera et al.,[SUP] [/SUP]who showed an increase of mitochondrial swelling and electron-lucent matrix, a known feature of ROS oxidative damage, induced by AAS in the liver of trained rats.
In conclusion, the results suggest that AAS could be a possible new risk factor for TAFLD. In this type of fatty liver disease, the individuals had a low body fat mass and they did not present IR.

