guardianactual
MuscleChemistry Registered Member
INTRODUCTION The ability of a hormone to elicit a biological effect in vivo depends on many factors including the affinity for its receptor and the rate at which it is cleared from the circulation. Some hormones, like atrial natriuretic peptide, have a very high affinity for their receptor (10 pM) and are cleared very rapidly (t1/2 ∼0.5 min) by receptor and protease-mediated events (1). Other hormones, like human growth hormone (hGH),1 have lower affinity for their receptor (300 pM) but are cleared more slowly (t1/2 ∼30 min in rats), primarily via the kidney (2, 3). Understanding the relationships between hormone affinity, clearance, and efficacy is important in optimizing hormone therapy. To study this systematically one would like to vary these parameters and evaluate their relative importance in regulating biopotency. hGH is a good model system in this regard as much is known about its structure and function (for review see Ref. 4). Simple receptor binding (5, 6), cell-based assays (7, 8), and growth parameters in rodents (9) can be used to determine biopotency in vitro and in vivo. The properties of proteins such as hGH that are cleared by kidney filtration can be modulated by attachment of polyethylene glycol (PEG) polymers, which increases the hydrodynamic volume of the hormone and thereby slows its clearance (10, for recent review see Ref. 11).
</br>
</br> Here, we describe a set of hGH derivatives conjugated with increasing numbers of PEG5000 polymers. The number and locations of modified amines were characterized as well as the effects on receptor binding kinetics and affinity. We also studied the circulating half-lives and in vivo potencies for PEG-hGH derivatives. We find that despite huge reductions in receptor on-rate and affinity, the efficacy of these analogs in vivo increases with increasing level of PEG modification and reaches an optimum at five PEG5000 groups per hGH. Thus, to a point, increasing circulating half-life can overcome the deficits in receptor binding affinity. Such analogs may be useful as long-acting alternatives to daily injections of hGH for treating growth hormone deficiency in children and in adults.
</br>
</br> EXPERIMENTAL PROCEDURES Materials Clinical grade recombinant hGH and hIGF-I were produced and provided by Genentech. The monomethyl ether (low diol) of PEG5000 was from Union Carbide; DCC and NHS were from Aldrich. Human GH binding protein (hGHbp) was produced in Escherichia coli. Preparation and Purification of PEG-hGH Derivatives PEG5000-monocarboxylic acid was prepared from the PEG5000-monomethyl ether by reaction with DCC and NHS in ethyl acetate to provide PEG5000-NHS as described (12). Briefly, the acid of PEG5000 was purified by dissolution in warm ethanol (1 g per 20 ml) and crystallized by cooling slowly to 4°C. The acid was filtered, washed three times with cold diethyl ether, and dried in vacuo. The pure acid (15 g, 3 mmol) was dissolved in ethyl acetate (150 ml) by warming, and NHS (0.86 g, 7.5 mmol) and DCC (1.55 g, 7.5 mmol) were added. The solution was stirred for 18 h at 30°C. Occasionally, the product precipitated during the reaction, in which case the white suspension was warmed until only the flocculent dicyclohexylurea remained undissolved. The latter was removed by filtration through Celite® and the solution cooled to 4°C for 20 h to precipitate the PEG5000-NHS product. This was collected by filtration, washed three times with cold ethyl acetate, and dried in vacuo to give 14.7 g of PEG5000-NHS.
</br>
</br> Recombinant hGH (10 mg/ml, in 0.05 M sodium borate buffer (pH 8.5)) was reacted for 30-60 min at room temperature with 1-3 eq of PEG5000-NHS per amino group on hGH (a total of 9 lysines plus the α-amine). After the reaction, buffer was added that contained 1.4 M sodium citrate, 0.05 M Tris (pH 7.5) to a final citrate concentration of 0.35 M. The mixture of PEG/hGH products was loaded onto a phenyl TSK 5PW column (1.6 × 40 cm) at a concentration of 2.75 mg of protein/ml of resin. The column was loaded at a flow rate of 45 cm/h and eluted with a reverse salt gradient of 0.35 M sodium citrate, 0.05 M Tris (pH 7.5) to 0.05 M Tris (pH 7.5) at a flow rate of 60 cm/h for 7 column volumes total. Fractions containing PEG-hGH species were pooled and concentrated 5-10-fold by ultrafiltration in a Centricon 10 concentrator (Amicon) or a Filtron 5K Omega 150-ml concentrator (Filtron). The concentrated protein was exchanged into 25 mM sodium acetate (pH 4.0) on a G-25 Sephadex column (Pharmacia Biotech Inc.).
</br>
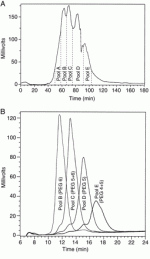
</br> The different PEG-hGH species were separated on a sulfopropyl-Sepharose high performance column (1.6 × 26 cm, Pharmacia) equilibrated in 25 mM sodium acetate (pH 4.0) at a concentration of 2.1 mg of protein/ml of resin (Fig. 1A). The PEGylated hGH derivatives were eluted in 7 column volumes using a salt gradient ranging from 0 to 0.3 M NaCl in 25 mM sodium acetate (pH 4.0) at 40 cm/h. Individual peaks were pooled, concentrated by ultrafiltration, and buffer exchanged using a PD-10 column (Pharmacia) equilibrated in 5 mM sodium phosphate, 18 mg/ml mannitol, and 0.68 mg/ml glycine (pH 7.4).
</br>
</br> Here, we describe a set of hGH derivatives conjugated with increasing numbers of PEG5000 polymers. The number and locations of modified amines were characterized as well as the effects on receptor binding kinetics and affinity. We also studied the circulating half-lives and in vivo potencies for PEG-hGH derivatives. We find that despite huge reductions in receptor on-rate and affinity, the efficacy of these analogs in vivo increases with increasing level of PEG modification and reaches an optimum at five PEG5000 groups per hGH. Thus, to a point, increasing circulating half-life can overcome the deficits in receptor binding affinity. Such analogs may be useful as long-acting alternatives to daily injections of hGH for treating growth hormone deficiency in children and in adults.
</br>
</br> EXPERIMENTAL PROCEDURES Materials Clinical grade recombinant hGH and hIGF-I were produced and provided by Genentech. The monomethyl ether (low diol) of PEG5000 was from Union Carbide; DCC and NHS were from Aldrich. Human GH binding protein (hGHbp) was produced in Escherichia coli. Preparation and Purification of PEG-hGH Derivatives PEG5000-monocarboxylic acid was prepared from the PEG5000-monomethyl ether by reaction with DCC and NHS in ethyl acetate to provide PEG5000-NHS as described (12). Briefly, the acid of PEG5000 was purified by dissolution in warm ethanol (1 g per 20 ml) and crystallized by cooling slowly to 4°C. The acid was filtered, washed three times with cold diethyl ether, and dried in vacuo. The pure acid (15 g, 3 mmol) was dissolved in ethyl acetate (150 ml) by warming, and NHS (0.86 g, 7.5 mmol) and DCC (1.55 g, 7.5 mmol) were added. The solution was stirred for 18 h at 30°C. Occasionally, the product precipitated during the reaction, in which case the white suspension was warmed until only the flocculent dicyclohexylurea remained undissolved. The latter was removed by filtration through Celite® and the solution cooled to 4°C for 20 h to precipitate the PEG5000-NHS product. This was collected by filtration, washed three times with cold ethyl acetate, and dried in vacuo to give 14.7 g of PEG5000-NHS.
</br>
</br> Recombinant hGH (10 mg/ml, in 0.05 M sodium borate buffer (pH 8.5)) was reacted for 30-60 min at room temperature with 1-3 eq of PEG5000-NHS per amino group on hGH (a total of 9 lysines plus the α-amine). After the reaction, buffer was added that contained 1.4 M sodium citrate, 0.05 M Tris (pH 7.5) to a final citrate concentration of 0.35 M. The mixture of PEG/hGH products was loaded onto a phenyl TSK 5PW column (1.6 × 40 cm) at a concentration of 2.75 mg of protein/ml of resin. The column was loaded at a flow rate of 45 cm/h and eluted with a reverse salt gradient of 0.35 M sodium citrate, 0.05 M Tris (pH 7.5) to 0.05 M Tris (pH 7.5) at a flow rate of 60 cm/h for 7 column volumes total. Fractions containing PEG-hGH species were pooled and concentrated 5-10-fold by ultrafiltration in a Centricon 10 concentrator (Amicon) or a Filtron 5K Omega 150-ml concentrator (Filtron). The concentrated protein was exchanged into 25 mM sodium acetate (pH 4.0) on a G-25 Sephadex column (Pharmacia Biotech Inc.).
</br>
</br> The different PEG-hGH species were separated on a sulfopropyl-Sepharose high performance column (1.6 × 26 cm, Pharmacia) equilibrated in 25 mM sodium acetate (pH 4.0) at a concentration of 2.1 mg of protein/ml of resin (Fig. 1A). The PEGylated hGH derivatives were eluted in 7 column volumes using a salt gradient ranging from 0 to 0.3 M NaCl in 25 mM sodium acetate (pH 4.0) at 40 cm/h. Individual peaks were pooled, concentrated by ultrafiltration, and buffer exchanged using a PD-10 column (Pharmacia) equilibrated in 5 mM sodium phosphate, 18 mg/ml mannitol, and 0.68 mg/ml glycine (pH 7.4).