drtbear1967
Musclechemistry Board Certified Member
[FONT="][h=4]NPB = MPS – MPB[/h]So what does this equation mean?
Net Protein Balance (skeletal muscle mass, for our purposes here) = Muscle Protein Synthesis – Muscle Protein Breakdown.
Net protein balance (NPB) is defined as muscle protein synthesis (MPS) minus muscle protein breakdown (MPB), or NPB = MPS – MPB. Thus, a significant rise in skeletal MPS (anabolism) and/or reduction in MPB (catabolism), such that NPB remains positive can result in increased skeletal muscle mass accretion.
Make it a positive value and you’re on your way to hugeness.
[h=4]Remodeling Muscle[/h]You have to eat right to build your muscle tissue back up after destroying it in the gym. That’s Bodybuilding 101. Under normal conditions, skeletal muscle has a high turnover rate – in the range of 1-2% of muscle proteins are being synthesized and broken down daily.
Both training and nutrient intake are potent activators of protein synthesis, although nutrient-induced increases are short-lived.
Training has a bigger effect; protein synthesis is ramped up for 24 hours in trained people.
The problem is that training also activates muscle protein degradation. Without the right nutrition at the right time, any potential muscle gain from increased protein synthesis could be canceled out by protein breakdown.
You can see how this works in the figure below. Without a training stimulus, muscle protein synthesis and muscle protein breakdown cancel each other out.
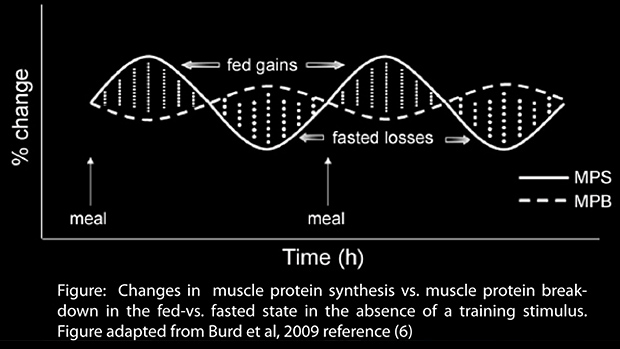
But add in an intense training session with the right nutrient intake at the right time and things change; protein synthesis is activated and degradation is suppressed. The result is an accumulation of muscle protein over time, as shown in the figure below.

[h=4]Protein Synthesis Primer: It’s ALL about mTOR[/h]To understand protein synthesis, it’s important to become better acquainted with mTor. Research tells us that when you force a muscle to contract against a heavy load, the primary response is an activation of protein synthesis. Protein synthesis activation is, in turn, controlled by a series of phosphorylation events orchestrated by a protein called mammalian target of rapamycin, or mTOR for short.
mTOR is arguably the most important cell signaling complex for muscle growth. It’s the master-controller of protein synthesis in the cell, and there’s a direct relationship between muscle growth and mTOR activation; the more a workout activates mTOR, the more the protein synthesis machinery cranks out new proteins for muscle growth and repair.
mTOR is activated by three things:
You take advantage of the Anabolic Window. To get as big as possible you must exploit the window for maximal effect. It’s time to talk about what to eat, and when.
There are three times for increasing protein/amino acid availability to augment the acute increase in protein synthesis caused by training:
Scientists have looked into this, and the results of several studies are shown in the figure below.
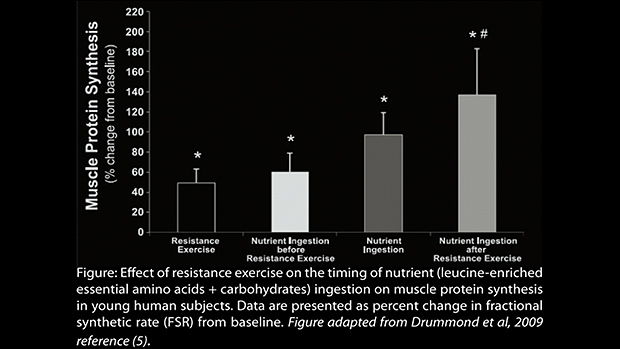
The take-home from this chart is that post-workout nutrition amplifies the acute, exercise induced increase in protein synthesis more than pre-workout nutrition. This is good information to know, but there’s much more to this story.
[h=4]Pre-workout[/h]During training, ATP is burned to fuel muscle contractions, which increases AMP levels. This activates a protein called AMP kinase (AMPK). AMPK reduces protein synthesis by inhibiting mTOR.
Think of it like this – if mTOR is like the gas-pedal for protein synthesis, then AMPK is the brakes. While it’s been shown that pre-workout nutrition doesn’t improve the post-workout burst in protein synthesis better than exercise alone, pre-workout amino acid intake does blunt AMPK mediated inhibition of mTOR.
Take home point: Don’t forget about pre-workout nutrition. It keeps the protein synthesis machinery from getting turned off during the workout.
[h=4]Peri-workout[/h]Researchers have also compared the effects of peri-workout nutrition to post-workout nutrition on protein synthesis. The results of these studies are similar to the pre-workout studies in that protein intake during a strength training workout resulted in an increase in protein synthesis, but much less-so than when protein was delivered post-workout.
While peri-workout amino acids have a subtle effect on protein synthesis, protein intake still causes an insulin response. This is important, because insulin is a powerful inhibitor of protein degradation.
It also makes a good case to include carbs peri-workout. Not only have peri-workout carbs been shown to inhibit protein degradation, but they also blunt AMPK mediated inhibition of mTOR.
Take home point: Peri-workout carbs not only inhibit protein degradation, but they also help to keep the protein synthetic machinery on during the workout.
[h=4]Post-workout[/h]The post-workout meal is the most important for amping up protein synthesis after a workout. Muscle cells are primed for protein synthesis in the hours after training, but only if the right nutrition is there.
To make more muscle we need protein, and the type and timing of protein intake during the post-workout period has been shown to control the overall increase in protein synthesis that occurs immediately after training.
Importantly, activation of protein synthesis in the short term seems to ultimately determine how well we respond to training in the long term. What this means is that not only are intense workouts needed to maximally activate protein synthesis, but the right nutrition needs to be there at precisely the right time for this to happen.
The window is only open for a short time, and long term gains in muscle can be compromised if protein intake is delayed for as little as two hours after training. Hit this window just right, and you’ll grow a heck of a lot more – miss it, and you may not grow at all!
There’s been considerable research on exactly what type of nutrition is needed to maximally activate protein synthesis. While we’ll discuss specifics later, it’s important to know that only the essential amino acids (EAAs) have been shown to activate protein synthesis, with leucine in particular being the most important for turning on the protein synthesis machinery.
It’s also clear from the literature that carbs aren’t needed to activate protein synthesis after training, but there are other reasons to include carbs, which we’ll get into later.
[h=4]So How Much Protein?[/h]It would be great if we could simply inhale 1000 grams of protein or amino acids pre, post, or peri-workout, and then grow as much as we want. Unfortunately, this would at best get converted to triglyceride and turned into bodyfat.
Proteins act synergistically with weight training to stimulate protein synthesis, but just as there’s an upper limit to how much exercise we can productively recover from, there also appears to be an upper limit to how much protein we can eat to max out protein synthesis.
This subject has been studied numerous times, but the amount of protein or amino acids used in the research may not directly apply to real-world scenarios. Scientists have rarely used a training stimulus that comes even close to what most guys are doing in the gym, making it difficult to extrapolate and make specific recommendations as far as how much protein is needed.
For instance, one study found that whey protein-induced increases in protein synthesis post-resistance exercise peaked at 20 grams of protein, with larger amounts not increasing the response any further. Similar dose-response studies have been done to determine the maximal requirements for leucine.
It’s important to realize that the kind of intense, balls-out training most lifters do probably activates protein synthesis to a greater degree than what researchers are using in the lab. Therefore, it’s possible that more than 20 grams may be needed for most people to get a maximal response.
So what’s the optimal amount, and when? We can offer rough recommendations, but it’s important to experiment to find the right formula for you.
[h=4]The Case for Carbs[/h]It’s been shown conclusively in the literature that insulin signaling isn’t needed to turn on training-induced protein synthesis – just leucine is required, which suggests that carbs aren’t important.
This originally came as quite a surprise, because insulin is a potent activator of protein synthesis. Insulin activates mTOR by way of PI3K/akt signaling, which is parallel to the pathways used by amino acids and mechanical stress to activate mTOR.
Although insulin signaling may not be needed for that burst in protein synthesis that occurs in the hours after a workout, there’s more to the story. Insulin is also a powerful inhibitor of muscle protein degradation.
Studies have found that both local hyperinsulinemia and the ingestion of carbs inhibits protein breakdown, with little to no effect on protein synthesis. When this was looked at specifically in the post-workout period, it was found that post-workout glucose consumption, although not activating protein synthesis, also had a powerful inhibiting effect on protein degradation.
That doesn’t mean we should discount carbs as far as protein synthesis goes; they increase insulin levels, which may still be important. Muscles are primed for increased protein synthesis for 24+ hours after training, but the acute burst in protein synthesis that occurs as a result of training or amino acid intake only lasts for a few hours.
Mechanical stress from training, amino acid intake, and insulin/growth factors all activate mTOR through different but complimentary pathways, suggesting that if multiple mTOR activating pathways are turned on at the same time, we may be able to get a synergistic effect.
It’s well established that the mechanical stress from training and leucine/EAAs synergistically amplify protein synthesis. Likewise, insulin may contribute to the overall burst in protein synthesis by turning on mTOR through the PI3K/akt pathway.
Although some studies looking specifically at resistance exercise-induced protein synthesis have shown that the addition of carbs to amino acids doesn’t result in an additive effect on protein synthesis when ample amounts of amino acids are ingested, you have to look closely at the experimental model when applying research to the real world.
More recent studies looking at a more general model for protein synthesis show that insulin + amino acids can have a synergistically positive effect on protein synthesis, causing the greatest mTOR activation together!
Taking all this work together, it’s safe to say that while insulin doesn’t appear to increase exercise-induced protein synthesis, it may act to “hold the throttle open longer” for the protein synthetic machinery after a workout.
Naturally, if insulin is able to extend or amplify the post-workout burst in protein synthesis, there would be a huge advantage to including carbs as part of your post-workout plan.
[h=4]Putting it all together[/h]Studies and literature are the backbone of the scientific method, but it’s all worthless if you don’t have a practical means to apply that information.
With that in mind, here’s how to put this all into practice.
Pre-workout (30-60 minutes out)
Example 2 pre-workout meal: Whey protein isolate with approximately 45 grams carbs from 1/2 cup oatmeal mixed with 1/2 cup unsweetened applesauce.
Peri-workout: (during the workout)
For pre-contest trainees or those who are less insulin sensitive, there’s a fat-burning advantage to keeping insulin low, so some people may want to omit carbs here. For offseason lifters or true hardgainers, the insulin response can be very helpful.
Example 1 peri-workout meal: 30-50 grams of casein hydrolysates like MAG-10®, and if off-season add 40 grams of potato starch.
Example 2 peri-workout meal: 20 grams BCAAs, and if off-season also 40-50g carbs from dextrose/glucose polymers.
Post-workout (up to 60 minutes after training)
Use 25-75g of medium-to low GI carbs. Off-season lifters or hard-gainers may want to have 50-100g of a mixture of medium to high GI carbs.
True hard-gainers can really benefit from the protein degradation inhibiting effects of insulin here. The big spike in insulin from the high GI carbs and more sustained elevation from medium GI carbs may also keep the protein synthesis throttle open longer.
If you’re pre-contest or for less insulin-sensitive people, occasionally omit carbs altogether during this meal, but don’t make it a rule.
Example 1 post-workout meal: 50 grams of whey isolate 15 minutes after training; if off-season, mix in 1-2 cups of raw milk. One hour later consume fish and Ezekiel toast with jam.
Example 2 post-workout meal: 50 grams whey isolate; if off-season also 1 cup oatmeal with 1 cup blueberries. One hour later eat next regular meal.
[h=4]Wrap-up[/h]Nutrients have a potent effect on the protein synthetic machinery, and timing them right can make or break your training progress. While there’s no ideal, one-size fits all solution for everyone – that depends on individual insulin sensitivity, metabolism, body type, and goals – we’ve set you up with a peri-workout nutritional strategy based on the latest scientific research that can be easily modified to suit every lifter’s needs. Use it as a template to maximize protein synthesis and grow like never before.
References:
Baar K, Esser K. Phosphorylation of p70(S6k) correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol 1999;276:C120-C127.
Beelen M, Koopman R, Gijsen AP, Vandereyt H, Kies AK, Kuipers H, et al. Protein coingestion stimulates muscle protein synthesis during resistance-type exercise. Am J Physiol Endocrinol Metab 2008;295:E70-E77.
Biolo G, Tipton KD, Klein S, Wolfe RR. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol 1997;273:E122-E129.
Biolo G, Declan Fleming RY, Wolfe RR. Physiologic hyperinsulinemia stimulates protein synthesis and enhances transport of selected amino acids in human skeletal muscle. J Clin Invest 1995;95:811-9.
Biolo G, Williams BD, Fleming RY, Wolfe RR. Insulin action on muscle protein kinetics and amino acid transport during recovery after resistance exercise. Diabetes 1999;48:949-57.
Borsheim E, Aarsland A, Wolfe RR. Effect of an amino acid, protein, and carbohydrate mixture on net muscle protein balance after resistance exercise. Int J Sport Nutr Exerc Metab 2004;14:255-71.
Burd NA, Tang JE, Moore DR, Phillips SM. Exercise training and protein metabolism: influences of contraction, protein intake, and sex-based differences. J Appl Physiol 2009;106:1692-701.
Carraro F, Stuart CA, Hartl WH, Rosenblatt J, Wolfe RR. Effect of exercise and recovery on muscle protein synthesis in human subjects. Am J Physiol 1990;259:E470-E476.
Chesley A, MacDougall JD, Tarnopolsky MA, Atkinson SA, Smith K. Changes in human muscle protein synthesis after resistance exercise. J Appl Physiol 1992;73:1383-8.
Chow LS, Albright RC, Bigelow ML, Toffolo G, Cobelli C, Nair KS. Mechanism of insulin’s anabolic effect on muscle: measurements of muscle protein synthesis and breakdown using aminoacyl-tRNA and other surrogate measures. Am J Physiol Endocrinol Metab 2006;291:E729-E736.
Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, et al. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J 2005;19:422-4.
Dennis MD, Baum JI, Kimball SR, Jefferson LS. Mechanisms involved in the coordinate regulation of mTORC1 by insulin and amino acids. J Biol Chem 2011;286:8287-96.
Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB. Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol 2006;576:613-24.
Drummond MJ, Dreyer HC, Fry CS, Glynn EL, Rasmussen BB. Nutritional and contractile regulation of human skeletal muscle protein synthesis and mTORC1 signaling. J Appl Physiol 2009;106:1374-84.
Drummond MJ, Dreyer HC, Pennings B, Fry CS, Dhanani S, Dillon EL, et al. Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. J Appl Physiol 2008;104:1452-61.
Fryburg DA, Jahn LA, Hill SA, Oliveras DM, Barrett EJ. Insulin and insulin-like growth factor-I enhance human skeletal muscle protein anabolism during hyperaminoacidemia by different mechanisms. J Clin Invest 1995;96:1722-9.
Fujita S, Dreyer HC, Drummond MJ, Glynn EL, Cadenas JG, Yoshizawa F, et al. Nutrient signalling in the regulation of human muscle protein synthesis. J Physiol 2007;582:813-23.
Fujita S, Dreyer HC, Drummond MJ, Glynn EL, Volpi E, Rasmussen BB. Essential amino acid and carbohydrate ingestion before resistance exercise does not enhance postexercise muscle protein synthesis. J Appl Physiol 2009;106:1730-9.
Hardie DG, Sakamoto K. AMPK: a key sensor of fuel and energy status in skeletal muscle. Physiology (Bethesda ) 2006;21:48-60.
Hartman JW, Tang JE, Wilkinson SB, Tarnopolsky MA, Lawrence RL, Fullerton AV, et al. Consumption of fat-free fluid milk after resistance exercise promotes greater lean mass accretion than does consumption of soy or carbohydrate in young, novice, male weightlifters. Am J Clin Nutr 2007;86:373-81.
Moore DR, Robinson MJ, Fry JL, Tang JE, Glover EI, Wilkinson SB, et al. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am J Clin Nutr 2009;89:161-8.
MacDougall JD, Gibala MJ, Tarnopolsky MA, MacDonald JR, Interisano SA, Yarasheski KE. The time course for elevated muscle protein synthesis following heavy resistance exercise. Can J Appl Physiol 1995;20:480-6.
Miller BF, Olesen JL, Hansen M, Dossing S, Crameri RM, Welling RJ, et al. Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J Physiol 2005;567:1021-33.
Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol 1997;273:E99-107.
Proud CG. Regulation of protein synthesis by insulin. Biochem Soc Trans 2006;34:213-6.
Terzis G, Georgiadis G, Stratakos G, Vogiatzis I, Kavouras S, Manta P, et al. Resistance exercise-induced increase in muscle mass correlates with p70S6 kinase phosphorylation in human subjects. Eur J Appl Physiol 2008;102:145-52.
Tipton KD, Ferrando AA, Phillips SM, Doyle D, Jr., Wolfe RR. Postexercise net protein synthesis in human muscle from orally administered amino acids. Am J Physiol 1999;276:E628-E634.
Welle S, Thornton C, Statt M, McHenry B. Postprandial myofibrillar and whole body protein synthesis in young and old human subjects. Am J Physiol 1994;267:E599-E604.
Wong TS, Booth FW. Protein metabolism in rat gastrocnemius muscle after stimulated chronic concentric exercise. J Appl Physiol 1990;69:1709-17.
Wong TS, Booth FW. Protein metabolism in rat tibialis anterior muscle after stimulated chronic eccentric exercise. J Appl Physiol 1990;69:1718-24.
Roy BD, Tarnopolsky MA, MacDougall JD, Fowles J, Yarasheski KE. Effect of glucose supplement timing on protein metabolism after resistance training. J Appl Physiol 1997;82:1882-
[/FONT]
Net Protein Balance (skeletal muscle mass, for our purposes here) = Muscle Protein Synthesis – Muscle Protein Breakdown.
Net protein balance (NPB) is defined as muscle protein synthesis (MPS) minus muscle protein breakdown (MPB), or NPB = MPS – MPB. Thus, a significant rise in skeletal MPS (anabolism) and/or reduction in MPB (catabolism), such that NPB remains positive can result in increased skeletal muscle mass accretion.
Make it a positive value and you’re on your way to hugeness.
[h=4]Remodeling Muscle[/h]You have to eat right to build your muscle tissue back up after destroying it in the gym. That’s Bodybuilding 101. Under normal conditions, skeletal muscle has a high turnover rate – in the range of 1-2% of muscle proteins are being synthesized and broken down daily.
Both training and nutrient intake are potent activators of protein synthesis, although nutrient-induced increases are short-lived.
Training has a bigger effect; protein synthesis is ramped up for 24 hours in trained people.
The problem is that training also activates muscle protein degradation. Without the right nutrition at the right time, any potential muscle gain from increased protein synthesis could be canceled out by protein breakdown.
You can see how this works in the figure below. Without a training stimulus, muscle protein synthesis and muscle protein breakdown cancel each other out.

But add in an intense training session with the right nutrient intake at the right time and things change; protein synthesis is activated and degradation is suppressed. The result is an accumulation of muscle protein over time, as shown in the figure below.

[h=4]Protein Synthesis Primer: It’s ALL about mTOR[/h]To understand protein synthesis, it’s important to become better acquainted with mTor. Research tells us that when you force a muscle to contract against a heavy load, the primary response is an activation of protein synthesis. Protein synthesis activation is, in turn, controlled by a series of phosphorylation events orchestrated by a protein called mammalian target of rapamycin, or mTOR for short.
mTOR is arguably the most important cell signaling complex for muscle growth. It’s the master-controller of protein synthesis in the cell, and there’s a direct relationship between muscle growth and mTOR activation; the more a workout activates mTOR, the more the protein synthesis machinery cranks out new proteins for muscle growth and repair.
mTOR is activated by three things:
- Mechanical stress (from heavy training loads)
- Growth factors (IGF, growth hormone, insulin, etc.)
- Amino acids (particularly leucine)
You take advantage of the Anabolic Window. To get as big as possible you must exploit the window for maximal effect. It’s time to talk about what to eat, and when.
There are three times for increasing protein/amino acid availability to augment the acute increase in protein synthesis caused by training:
- Pre-workout: Within an hour or so before the workout begins.
- Peri-workout: During the training session.
- Post-workout: Less than two hours post-exercise.
Scientists have looked into this, and the results of several studies are shown in the figure below.

The take-home from this chart is that post-workout nutrition amplifies the acute, exercise induced increase in protein synthesis more than pre-workout nutrition. This is good information to know, but there’s much more to this story.
[h=4]Pre-workout[/h]During training, ATP is burned to fuel muscle contractions, which increases AMP levels. This activates a protein called AMP kinase (AMPK). AMPK reduces protein synthesis by inhibiting mTOR.
Think of it like this – if mTOR is like the gas-pedal for protein synthesis, then AMPK is the brakes. While it’s been shown that pre-workout nutrition doesn’t improve the post-workout burst in protein synthesis better than exercise alone, pre-workout amino acid intake does blunt AMPK mediated inhibition of mTOR.
Take home point: Don’t forget about pre-workout nutrition. It keeps the protein synthesis machinery from getting turned off during the workout.
[h=4]Peri-workout[/h]Researchers have also compared the effects of peri-workout nutrition to post-workout nutrition on protein synthesis. The results of these studies are similar to the pre-workout studies in that protein intake during a strength training workout resulted in an increase in protein synthesis, but much less-so than when protein was delivered post-workout.
While peri-workout amino acids have a subtle effect on protein synthesis, protein intake still causes an insulin response. This is important, because insulin is a powerful inhibitor of protein degradation.
It also makes a good case to include carbs peri-workout. Not only have peri-workout carbs been shown to inhibit protein degradation, but they also blunt AMPK mediated inhibition of mTOR.
Take home point: Peri-workout carbs not only inhibit protein degradation, but they also help to keep the protein synthetic machinery on during the workout.
[h=4]Post-workout[/h]The post-workout meal is the most important for amping up protein synthesis after a workout. Muscle cells are primed for protein synthesis in the hours after training, but only if the right nutrition is there.
To make more muscle we need protein, and the type and timing of protein intake during the post-workout period has been shown to control the overall increase in protein synthesis that occurs immediately after training.
Importantly, activation of protein synthesis in the short term seems to ultimately determine how well we respond to training in the long term. What this means is that not only are intense workouts needed to maximally activate protein synthesis, but the right nutrition needs to be there at precisely the right time for this to happen.
The window is only open for a short time, and long term gains in muscle can be compromised if protein intake is delayed for as little as two hours after training. Hit this window just right, and you’ll grow a heck of a lot more – miss it, and you may not grow at all!
There’s been considerable research on exactly what type of nutrition is needed to maximally activate protein synthesis. While we’ll discuss specifics later, it’s important to know that only the essential amino acids (EAAs) have been shown to activate protein synthesis, with leucine in particular being the most important for turning on the protein synthesis machinery.
It’s also clear from the literature that carbs aren’t needed to activate protein synthesis after training, but there are other reasons to include carbs, which we’ll get into later.

[h=4]So How Much Protein?[/h]It would be great if we could simply inhale 1000 grams of protein or amino acids pre, post, or peri-workout, and then grow as much as we want. Unfortunately, this would at best get converted to triglyceride and turned into bodyfat.
Proteins act synergistically with weight training to stimulate protein synthesis, but just as there’s an upper limit to how much exercise we can productively recover from, there also appears to be an upper limit to how much protein we can eat to max out protein synthesis.
This subject has been studied numerous times, but the amount of protein or amino acids used in the research may not directly apply to real-world scenarios. Scientists have rarely used a training stimulus that comes even close to what most guys are doing in the gym, making it difficult to extrapolate and make specific recommendations as far as how much protein is needed.
For instance, one study found that whey protein-induced increases in protein synthesis post-resistance exercise peaked at 20 grams of protein, with larger amounts not increasing the response any further. Similar dose-response studies have been done to determine the maximal requirements for leucine.
It’s important to realize that the kind of intense, balls-out training most lifters do probably activates protein synthesis to a greater degree than what researchers are using in the lab. Therefore, it’s possible that more than 20 grams may be needed for most people to get a maximal response.
So what’s the optimal amount, and when? We can offer rough recommendations, but it’s important to experiment to find the right formula for you.
[h=4]The Case for Carbs[/h]It’s been shown conclusively in the literature that insulin signaling isn’t needed to turn on training-induced protein synthesis – just leucine is required, which suggests that carbs aren’t important.
This originally came as quite a surprise, because insulin is a potent activator of protein synthesis. Insulin activates mTOR by way of PI3K/akt signaling, which is parallel to the pathways used by amino acids and mechanical stress to activate mTOR.
Although insulin signaling may not be needed for that burst in protein synthesis that occurs in the hours after a workout, there’s more to the story. Insulin is also a powerful inhibitor of muscle protein degradation.
Studies have found that both local hyperinsulinemia and the ingestion of carbs inhibits protein breakdown, with little to no effect on protein synthesis. When this was looked at specifically in the post-workout period, it was found that post-workout glucose consumption, although not activating protein synthesis, also had a powerful inhibiting effect on protein degradation.
That doesn’t mean we should discount carbs as far as protein synthesis goes; they increase insulin levels, which may still be important. Muscles are primed for increased protein synthesis for 24+ hours after training, but the acute burst in protein synthesis that occurs as a result of training or amino acid intake only lasts for a few hours.
Mechanical stress from training, amino acid intake, and insulin/growth factors all activate mTOR through different but complimentary pathways, suggesting that if multiple mTOR activating pathways are turned on at the same time, we may be able to get a synergistic effect.
It’s well established that the mechanical stress from training and leucine/EAAs synergistically amplify protein synthesis. Likewise, insulin may contribute to the overall burst in protein synthesis by turning on mTOR through the PI3K/akt pathway.
Although some studies looking specifically at resistance exercise-induced protein synthesis have shown that the addition of carbs to amino acids doesn’t result in an additive effect on protein synthesis when ample amounts of amino acids are ingested, you have to look closely at the experimental model when applying research to the real world.
More recent studies looking at a more general model for protein synthesis show that insulin + amino acids can have a synergistically positive effect on protein synthesis, causing the greatest mTOR activation together!
Taking all this work together, it’s safe to say that while insulin doesn’t appear to increase exercise-induced protein synthesis, it may act to “hold the throttle open longer” for the protein synthetic machinery after a workout.
Naturally, if insulin is able to extend or amplify the post-workout burst in protein synthesis, there would be a huge advantage to including carbs as part of your post-workout plan.
[h=4]Putting it all together[/h]Studies and literature are the backbone of the scientific method, but it’s all worthless if you don’t have a practical means to apply that information.
With that in mind, here’s how to put this all into practice.
Pre-workout (30-60 minutes out)
- Protein Source: 30-50g of any medium to fast-acting protein source. Whole-food is okay, but you may want to restrict whole-food protein closer to 60 minutes out than 30 minutes out. Examples of fast-acting protein sources include mixtures of whey and casein isolates/hydrolysates and concentrates.
- Carb Source: Optional, but if you plan to train hard, you should include carbs. 25-75g of low to medium GI carbs. Example is a cup of oatmeal with a cup of blueberries.
Example 2 pre-workout meal: Whey protein isolate with approximately 45 grams carbs from 1/2 cup oatmeal mixed with 1/2 cup unsweetened applesauce.
Peri-workout: (during the workout)
- Protein Source: 10-20g of BCAAs or 20-30g of isolates / hydrolysates from casein or whey or a mixture.
- Carb Source: Optional. 35-50g of high glycemic carbs, sipped throughout the workout.
For pre-contest trainees or those who are less insulin sensitive, there’s a fat-burning advantage to keeping insulin low, so some people may want to omit carbs here. For offseason lifters or true hardgainers, the insulin response can be very helpful.
Example 1 peri-workout meal: 30-50 grams of casein hydrolysates like MAG-10®, and if off-season add 40 grams of potato starch.
Example 2 peri-workout meal: 20 grams BCAAs, and if off-season also 40-50g carbs from dextrose/glucose polymers.
Post-workout (up to 60 minutes after training)
- Protein Source: 30-50g fast-acting protein: whey isolates/ hydrolysates or casein hydrolysate.
- Carb Source: Optional but highly advisable unless you are in a drastic fat reduction mode.
Use 25-75g of medium-to low GI carbs. Off-season lifters or hard-gainers may want to have 50-100g of a mixture of medium to high GI carbs.
True hard-gainers can really benefit from the protein degradation inhibiting effects of insulin here. The big spike in insulin from the high GI carbs and more sustained elevation from medium GI carbs may also keep the protein synthesis throttle open longer.
If you’re pre-contest or for less insulin-sensitive people, occasionally omit carbs altogether during this meal, but don’t make it a rule.
Example 1 post-workout meal: 50 grams of whey isolate 15 minutes after training; if off-season, mix in 1-2 cups of raw milk. One hour later consume fish and Ezekiel toast with jam.
Example 2 post-workout meal: 50 grams whey isolate; if off-season also 1 cup oatmeal with 1 cup blueberries. One hour later eat next regular meal.
[h=4]Wrap-up[/h]Nutrients have a potent effect on the protein synthetic machinery, and timing them right can make or break your training progress. While there’s no ideal, one-size fits all solution for everyone – that depends on individual insulin sensitivity, metabolism, body type, and goals – we’ve set you up with a peri-workout nutritional strategy based on the latest scientific research that can be easily modified to suit every lifter’s needs. Use it as a template to maximize protein synthesis and grow like never before.
References:
Baar K, Esser K. Phosphorylation of p70(S6k) correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol 1999;276:C120-C127.
Beelen M, Koopman R, Gijsen AP, Vandereyt H, Kies AK, Kuipers H, et al. Protein coingestion stimulates muscle protein synthesis during resistance-type exercise. Am J Physiol Endocrinol Metab 2008;295:E70-E77.
Biolo G, Tipton KD, Klein S, Wolfe RR. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol 1997;273:E122-E129.
Biolo G, Declan Fleming RY, Wolfe RR. Physiologic hyperinsulinemia stimulates protein synthesis and enhances transport of selected amino acids in human skeletal muscle. J Clin Invest 1995;95:811-9.
Biolo G, Williams BD, Fleming RY, Wolfe RR. Insulin action on muscle protein kinetics and amino acid transport during recovery after resistance exercise. Diabetes 1999;48:949-57.
Borsheim E, Aarsland A, Wolfe RR. Effect of an amino acid, protein, and carbohydrate mixture on net muscle protein balance after resistance exercise. Int J Sport Nutr Exerc Metab 2004;14:255-71.
Burd NA, Tang JE, Moore DR, Phillips SM. Exercise training and protein metabolism: influences of contraction, protein intake, and sex-based differences. J Appl Physiol 2009;106:1692-701.
Carraro F, Stuart CA, Hartl WH, Rosenblatt J, Wolfe RR. Effect of exercise and recovery on muscle protein synthesis in human subjects. Am J Physiol 1990;259:E470-E476.
Chesley A, MacDougall JD, Tarnopolsky MA, Atkinson SA, Smith K. Changes in human muscle protein synthesis after resistance exercise. J Appl Physiol 1992;73:1383-8.
Chow LS, Albright RC, Bigelow ML, Toffolo G, Cobelli C, Nair KS. Mechanism of insulin’s anabolic effect on muscle: measurements of muscle protein synthesis and breakdown using aminoacyl-tRNA and other surrogate measures. Am J Physiol Endocrinol Metab 2006;291:E729-E736.
Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, et al. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J 2005;19:422-4.
Dennis MD, Baum JI, Kimball SR, Jefferson LS. Mechanisms involved in the coordinate regulation of mTORC1 by insulin and amino acids. J Biol Chem 2011;286:8287-96.
Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB. Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol 2006;576:613-24.
Drummond MJ, Dreyer HC, Fry CS, Glynn EL, Rasmussen BB. Nutritional and contractile regulation of human skeletal muscle protein synthesis and mTORC1 signaling. J Appl Physiol 2009;106:1374-84.
Drummond MJ, Dreyer HC, Pennings B, Fry CS, Dhanani S, Dillon EL, et al. Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. J Appl Physiol 2008;104:1452-61.
Fryburg DA, Jahn LA, Hill SA, Oliveras DM, Barrett EJ. Insulin and insulin-like growth factor-I enhance human skeletal muscle protein anabolism during hyperaminoacidemia by different mechanisms. J Clin Invest 1995;96:1722-9.
Fujita S, Dreyer HC, Drummond MJ, Glynn EL, Cadenas JG, Yoshizawa F, et al. Nutrient signalling in the regulation of human muscle protein synthesis. J Physiol 2007;582:813-23.
Fujita S, Dreyer HC, Drummond MJ, Glynn EL, Volpi E, Rasmussen BB. Essential amino acid and carbohydrate ingestion before resistance exercise does not enhance postexercise muscle protein synthesis. J Appl Physiol 2009;106:1730-9.
Hardie DG, Sakamoto K. AMPK: a key sensor of fuel and energy status in skeletal muscle. Physiology (Bethesda ) 2006;21:48-60.
Hartman JW, Tang JE, Wilkinson SB, Tarnopolsky MA, Lawrence RL, Fullerton AV, et al. Consumption of fat-free fluid milk after resistance exercise promotes greater lean mass accretion than does consumption of soy or carbohydrate in young, novice, male weightlifters. Am J Clin Nutr 2007;86:373-81.
Moore DR, Robinson MJ, Fry JL, Tang JE, Glover EI, Wilkinson SB, et al. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am J Clin Nutr 2009;89:161-8.
MacDougall JD, Gibala MJ, Tarnopolsky MA, MacDonald JR, Interisano SA, Yarasheski KE. The time course for elevated muscle protein synthesis following heavy resistance exercise. Can J Appl Physiol 1995;20:480-6.
Miller BF, Olesen JL, Hansen M, Dossing S, Crameri RM, Welling RJ, et al. Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J Physiol 2005;567:1021-33.
Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol 1997;273:E99-107.
Proud CG. Regulation of protein synthesis by insulin. Biochem Soc Trans 2006;34:213-6.
Terzis G, Georgiadis G, Stratakos G, Vogiatzis I, Kavouras S, Manta P, et al. Resistance exercise-induced increase in muscle mass correlates with p70S6 kinase phosphorylation in human subjects. Eur J Appl Physiol 2008;102:145-52.
Tipton KD, Ferrando AA, Phillips SM, Doyle D, Jr., Wolfe RR. Postexercise net protein synthesis in human muscle from orally administered amino acids. Am J Physiol 1999;276:E628-E634.
Welle S, Thornton C, Statt M, McHenry B. Postprandial myofibrillar and whole body protein synthesis in young and old human subjects. Am J Physiol 1994;267:E599-E604.
Wong TS, Booth FW. Protein metabolism in rat gastrocnemius muscle after stimulated chronic concentric exercise. J Appl Physiol 1990;69:1709-17.
Wong TS, Booth FW. Protein metabolism in rat tibialis anterior muscle after stimulated chronic eccentric exercise. J Appl Physiol 1990;69:1718-24.
Roy BD, Tarnopolsky MA, MacDougall JD, Fowles J, Yarasheski KE. Effect of glucose supplement timing on protein metabolism after resistance training. J Appl Physiol 1997;82:1882-
[/FONT]

