HackTwat
MuscleChemistry Registered Member
A UK Epidemic of Testosterone Prescribing</ARTICLE-TITLE> 2001-2010
Earn H. Gan, Stewart Pattman, Simon H. S. Pearce, Richard Quinton
Clin Endocrinol. 2013;79(4):564-570.
Abstract
Context Testosterone replacement therapy is the standard treatment for male hypogonadism. There has lately been increased marketing in the medical media promoting testosterone replacement for men with erectile dysfunction or for older men with low serum testosterone, despite the lack of long-term safety and efficacy data. Therefore, we aimed to examine trends in testosterone prescribing in UK primary care over the last 10 years.
Methods Data about the use of testosterone preparations from the Departments of Health Prescription Cost Analysis for community pharmacies 2001–2010, for England, Scotland and Wales, were collated. Community requests for serum total testosterone assay in men to the Biochemistry Department at the Newcastle upon Tyne Hospitals Trust were also examined over the same time period.
Results The number of prescriptions for testosterone preparations increased by nearly 90% from 157 602 to 298 134 dispensed items annually, over a 10-year period. However, due to a particularly significant (fivefold) increase in prescribing of (more expensive) transdermal preparations, the cost to the NHS showed a 267% escalation, from £3·2 to £11·7 million, annually over the same period.
Local requests from primary care in the Newcastle and North Tyneside area for serum testosterone measurement in men also increased, from 347 requests in 2000 to 823 requests in 2010, a 137% increase. However, the number of men with likely unequivocal hypogonadism (testosterone less than 6·0 nm) remained constant at 5·2% in 2000 and 6·3% in 2010.
Conclusion Many men in the UK might be receiving testosterone replacement therapy with neither clearly established indications nor robustly diagnosed hypogonadism. A national registry for men treated with testosterone and further evidence to improve current guidance (national and/or international) on the indications for testosterone replacement would be beneficial.
Background
Human efforts to restore virility and to reverse the effects of ageing in men have been documented since ancient times. As early as 1849, Adolph Berthold postulated a connection between testicular secretions and male behavioural and sexual characteristics from his testicular transplantation experiments. [SUP][1][/SUP] When testosterone was chemically synthesized in 1935, it marked the beginning of a new era in men's health. While the only validated indication for testosterone treatment is male hypogonadism with pathological androgen deficiency, there has been an increasing focus on its role as a 'function-promoting' therapy in older or obese men who wish to restore virility or male character, despite the lack of sound clinical evidence to support this practice.
Male hypogonadism is a clinical syndrome resulting from failure of the testis to produce physiological levels of testosterone and/or maintain normal spermatogenesis, due to disruption of one or more levels of the hypothalamic–pituitary–testis (HPT) axis. Features of hypogonadism include sexual dysfunction, loss of muscle bulk, central obesity, fatigue and mood/sleep disturbances. Long-term complications such as osteoporosis, low-impact fracture and chronic anaemia occur in untreated cases. [SUP][2][/SUP] Although these features are all associated with low serum testosterone concentration, not all men with a low serum testosterone have organic hypogonadism, and there is a large symptom overlap with obesity and nonendocrine illness. For instance, obesity is associated with progressively lower total and free serum testosterone independent of simultaneous reductions in sex hormone binding globulin (SHBG). [SUP][3][/SUP] Lower luteinizing hormone (LH) levels are also seen in obese men, suggesting a potential failure in the hypothalamus–pituitary level. [SUP][3][/SUP] Additionally, the HPT axis exhibits diurnal variation in activity and tends to physiologically shutdown with any form of acute stress, including strenuous physical exertion and sleep deprivation, [SUP][4][/SUP] or chronic disease, such as depression, malnutrition and obesity. There is even an acute suppressive effect of oral glucose loading. [SUP][5][/SUP] Importantly, the HPT axis invariably recovers once these modifiable factors are corrected, [SUP][6][/SUP] hence our preferred term 'functional' hypogonadotropic hypotestosteronaemia (FHH) of nongonadal illness.
Chronic use of prescription and/or nonprescription drugs can also induce hypogonadism, mostly through HPT axis suppression, including opioids, [SUP][7][/SUP] tricyclic antidepressants and selective serotonin reuptake inhibitors, [SUP][8][/SUP] antidopaminergic drugs with hyperprolactinaemic effect such as phenothiazines [SUP][8][/SUP] and risperidone, [SUP][9][/SUP] and antiandrogens such as spironolactone, finasteride [SUP][10][/SUP] and synthetic androgens/anabolic steroids. [SUP][11][/SUP] Combined primary and secondary hypogonadism is seen in some men with a history of excessive alcohol or cannabinoid consumption. [SUP][12, 13][/SUP] A recent double-blind randomized placebo-controlled study (RCT) demonstrated that pioglitazone significantly reduced total and (to an even greater degree) free testosterone levels in eugonadal men with type 2 diabetes. [SUP][14][/SUP]
Hence, testosterone replacement therapy (TRT) may not be appropriate for men with mild, functional and/or transient nonsyndromic hypotestosteronaemia, termed by us FHH, particularly older men with multiple comorbidities for whom treating the primary problem would be more appropriate. Nevertheless, despite the lack of long-term safety data and the inconsistent findings of case–control studies and small RCTs involving older men, [SUP][15–18][/SUP] there have lately been (to our eyes) aggressive marketing campaigns promoting TRT, targeting both the general public and primary care physicians who have sought to equate erectile dysfunction or 'hypogonadism' with 'low serum testosterone'. Therefore, we have set out to uncover the trend and the changes in testosterone prescribing in the primary care setting over the past 10 years.
Methods
Trends in Testosterone Hormone Prescribing: England, Scotland and Wales, 2000–2010
Data on the prescribing trends of testosterone therapy in England, Scotland and Wales were collated by analysing prescription cost analysis (PCA) statistics, data published annually by the Department of Health (DoH). Available data comprised the number of dispensed items from community pharmacists in these regions and the net ingredient costs. These data are based on information obtained from prescriptions sent to the NHS Prescription Pricing Division for payment and include prescriptions dispensed in the community by general practitioners, pharmacists, appliance contractors, prescriptions from hospital doctors dispensed in the community and items personally administered by doctors. We analysed PCA data for England for the calendar years 2000–2010, inclusive and for 2001–2010, inclusive for Scotland and Wales. The trend in testosterone usage on a year-by-year basis can be ascertained by analysing the PCA data. For testosterone products () introduced to the market after the year 2001 ( e.g. testosterone gel preparations and Nebido injection), we included the first full year of availability as the baseline in our analysis (e.g. we used cumulative data from years 2003–2004 as the baseline for Testogel, the lead testosterone in the UK, which was introduced in 2003).
Table 1. Testosterone products and cost per month according to product types
<TBODY>
</TBODY>*Net ingredient cost (NIC) per quantity is cited from British National Formulary (BNF) 64 (September 2012) and Prescription Analysis Data, England, 2011 Product information (formulation) is quoted from Home - electronic Medicines Compendium (eMC) and BNF 64 (September 2012).
Local Community Requests for Serum Total Testosterone Assay in Men, 2000–2010
The number of primary care requests from 2000 to 2010, inclusive, for total testosterone assay in men to the Biochemistry Department at the Newcastle upon Tyne Hospitals Trust (NuTH) was examined by interrogating the laboratory database using the Cognos Impromptu software (version 7.1, IBM Software, Armonk, USA). During this period, total testosterone was quantified using an automated competitive immunoassay. NuTH comprises hospitals delivering secondary care to the Tyneside area, serving a primary care population of 310 000 between 52 GP practices, and tertiary care across North East England and Cumbria. Our survey aimed to explore the trend in the number of serum testosterone assays for men requested by Tyneside GPs and the pattern of results generated from these requests.
Results
Analysis of Testosterone Hormone Usage in England (2000–2010), Wales and Scotland (2001–2010)
The number of prescription items dispensed for all forms of testosterone preparations in England, Wales and Scotland has increased by nearly 90% from 157 602 to 298 134 prescriptions, between 2001 and 2010.(Fig. 1 panels a & b). The cost of this medication to the NHS showed a remarkable 267% escalation, from £3·2 to £11·7 million over the same period. (Fig. 1, panels c & d). The trend in testosterone prescribing for all three regions is highly congruent, seeing a marked increase in the amount of prescriptions for both transdermal preparations and testosterone undecanoate injection. The rising pattern was most significant for transdermal preparations with a fivefold increase, from 28 247 to 151 676 dispensed items, and a 360% escalation in annual expenditure (£1·46 million–£6·74 million, 2001–2010) (Fig. 2).
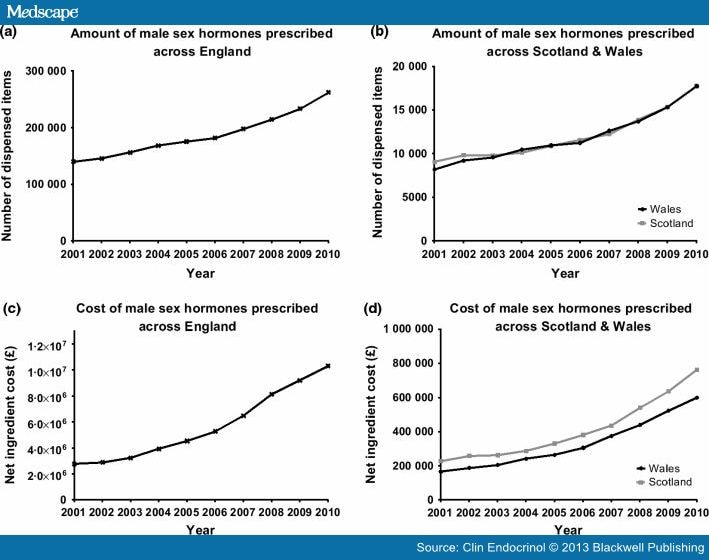
Figure 1.
Quantity and cost of testosterone prescribing in England, Wales and Scotland. Panel a & b: The number of prescription items for all testosterone replacement therapies, from 2001 to 2010. Panel c & d: Net ingredient cost for testosterone replacement, from 2001 to 2010.
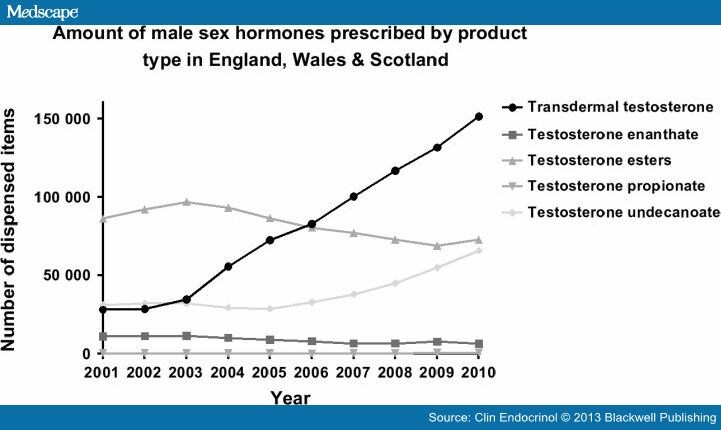
Figure 2.
The number of prescribed items for testosterone replacement according to subtypes in England, Wales and Scotland, 2001–2010.
When transdermal preparation prescriptions were analysed according to their subtypes, a significant sharp rise was observed in testosterone gel since their introduction in 2003, with a 3-fold increase, from 32 300 to 105 300 dispensed items, and 238% escalation in annual expenditure (£1·3 million–£4·54 million; year 2003/2004–2010), as per PCA data in England. On the other hand, the use of some other testosterone preparations, including transdermal patches, mucoadhesive buccal tablets and pellets for subcutaneous implant declined steadily after the introduction of transdermal testosterone gel products.
Of the three currently available testosterone gel products (Testim® [Ferring Pharmaceuticals, West Drayton, UK], Tostran-2%® [ProStrakan, Galashiels, UK] and Testogel® [Bayer Schering, Newbury, Berkshire, UK]), the latter is the most commonly prescribed product, representing 80% of testosterone gel prescribed (2010), constituting 64% of the total expenditure for all types of testosterone in 2010. The overall usage of gels has increased from 32 300–84 900 dispensed items (163%) since the 12-month period following its introduction of the lead product (Testogel®) in 2003.
The PCA data in England also revealed that the use of testosterone undecanoate has been on the rise over the past 10 years, from 31 336 to 65 998 items (111%; 2000–2010). This is solely attributed to the introduction of long-acting testosterone depot injection, Nebido® (Bayer Schering), for which there has been a major rise in prescriptions since its introduction in 2005, with a 365% escalation in dispense items (8 200–38 100; 2005/2006–2010) and a 4·5-fold increase in overall expenditure over the same period (£735 500–£3·29 million). The oral preparation of testosterone undecanoate (Restandol capsule®, Merck Sharp & Dohme Limited, Hoddesdon, Herts, UK) showed a modest fall in the last 10 years (27 400–22 900 items; 2001–2010).
The use of short-acting testosterone injection (testosterone enanthate, testosterone ester and testosterone propionate) has remained relatively stable over the past decade. Sustanon® (Merck Sharp & Dohme Limited) 100 mg (testosterone ester) and Andropatch® (GlaxoSmithKline, Brentford, Middlesex, UK) (transdermal testosterone) have been withdrawn from the UK market since 2010.
Analysis of Local Community Requests for Serum Total Testosterone Assay in Men, 2000–2010
Local requests for serum testosterone measurement in men from primary care in the NuTH Biochemistry Department have increased from 347 requests in 2000 to 823 requests in 2010, with a 137% rise (Fig. 3, panel a). However, the number of men with a high likelihood of hypogonadism (testosterone less than 6·0 nm) remained relatively constant at 5·2% in 2000 and 6·3% in 2010. On the other hand, the number of men with total testosterone in the 6–8·9 nm range, where there is greater uncertainty in the diagnosis of hypogonadism, increased by over 2-fold, from 7·51% to 16·99%. (Fig. 3, panel b).
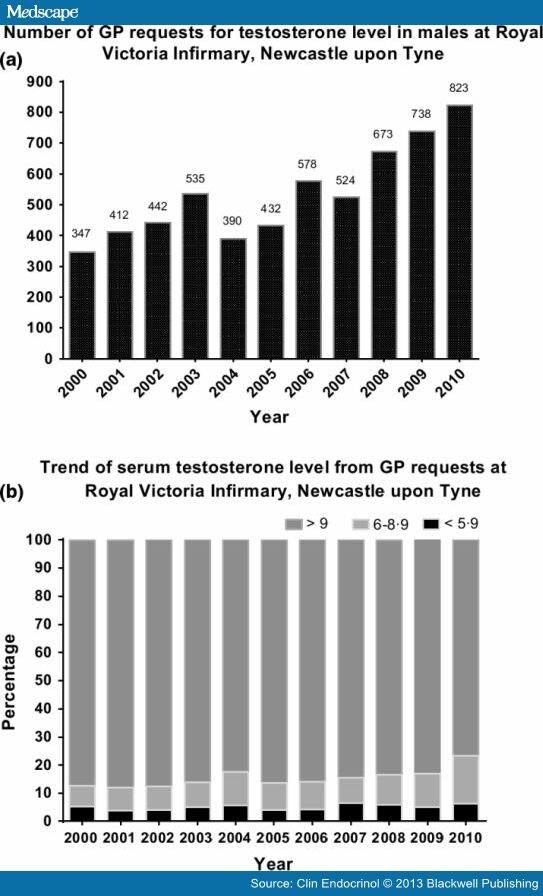
Figure 3.
Local requests for testosterone level in males at Royal Victoria Infirmary. Panel a. The number of GP requests for testosterone level. Panel b. The trend of serum testosterone level from the local requests.
Discussion
The PCA data from England, Wales and Scotland have clearly shown a progressive rise in the prescribing of testosterone from 2001–2010. This is in keeping with the data available globally, including Switzerland, [SUP][19][/SUP] United States [SUP][20][/SUP] and Australia, [SUP][21][/SUP] suggesting a potential 'pandemic' of testosterone prescribing. There are a number of potential explanations for this trend. Firstly, conditions associated with hypogonadism might be on the rise. For example, the birth prevalence per 100 000 men with Klinefelter syndrome (KS), the most common pathological cause of androgen deficiency, is reported as having risen from 109 in the 1960s–1970s to 223 during the period of 1986–2006. [SUP][22, 23][/SUP] There has been a definite increase in the number of childhood and adult cancer survivors, comprising men who have received radiotherapy to the pituitary or testis or have been orchidectomized for seminoma or teratoma of the testis. Finally, opioids such as tramadol and slow-released morphine sulphate tablets are now more widely used for the treatment of chronic noncancer pain. However, the nearly twofold increase in testosterone prescribing is highly unlikely to be solely attributable to an increased prevalence of male hypogonadism, especially as most men with KS, the commonest cause of pathological male hypogonadism, only become hypogonadal later in life. [SUP][24][/SUP]
Another possible explanation for the observed prescribing trend is increased testosterone testing in primary care, which could potentially result in either improved diagnosis of male hypogonadism and/or an unwarranted increase in TRT in men with borderline serum testosterone concentrations. It is entirely appropriate to treat symptomatic men with borderline-low testosterone under defined circumstances, such as gonadotrophin deficiency resulting from pituitary tumours or prescribed opiate analgesia, or elevated gonadotrophins diagnostic of primary testicular dysfunction ( e.g. KS). However, with these important exceptions, there is insufficient evidence for initiating TRT in most men with a borderline-low serum testosterone due to FHH. There are likewise no data to support testosterone therapy in the context of age-related frailty. [SUP][3, 25, 26][/SUP] We simply do not know whether borderline biochemical hypotestosteronaemia related to FHH is maladaptive, neutral or conceivably even adaptive.
Our local survey has demonstrated that the absolute number of men with likely unequivocal hypogonadism (testosterone <6·0 nm) has remained relatively constant despite a surge in testosterone testing in primary care. The rising number of requests has identified significantly more men with testosterone level in the 6–9 nm range, although 9 nm is the lower limit of the adult male normal range in our assay. Admittedly, this local pattern of requests and results might not reflect the overall situation in the UK, but there are currently no other comparable published data available.
Finally, a plausible explanation for the observed trend in testosterone prescribing in the UK is that an increasing number of eugonadal men might be receiving unnecessary testosterone therapy, particularly older men with 'nongonadal' illness. Although the newer testosterone preparations have undoubtedly improved the quality of life for many men with organic hypogonadism, due to ease of use and better pharmacokinetic profile, pharma has promoted TRT to a broader population of older men with sexual dysfunction. [SUP][27, 28][/SUP] Over the past 3 years, the advertising tracker Kantar Media has reported an increase of more than 170% in spending on advertising by pharmaceutical companies such as Abbott and Eli Lilly in the USA to promote TRT. [SUP][29][/SUP]
Although direct-to-consumer pharmaceutical marketing is not permitted in the UK, the public has virtually unfettered access to pharma websites as key sources of information relating to ageing, erectile dysfunction and the 'andropause'. [SUP][30–33][/SUP] A general theme from pharma-hosted online sources is that testosterone deficiency is common and particularly so with advancing age. Hence, it should be no surprise if men with nonspecific, physiological, age- or illness-related symptoms should increasingly consider these as possible manifestations of testosterone deficiency, with a consequent belief that TRT might restore their quality of life and virility. The question is whether this information campaign has over-reached the actual evidence?
It is well established that a significant portion of older men have testosterone levels below the lower limit for healthy, young men, with an average decline in serum testosterone levels of 1–2% per year. [SUP][34][/SUP] Although this has historically been attributed to physiological decline in testosterone production with age, a large 5-year longitudinal study involving 1 382 community-dwelling men reported that the testosterone decline seen in older men was actually largely caused by a combination of an increased burden of nonendocrine disease and modifiable factors, such as obesity and lifestyle, rather than being an inevitable fact of ageing per se. [SUP][35][/SUP] Indeed, the Odense Androgen Study demonstrated similar serum testosterone reference intervals for both young and elderly healthy men. [SUP][36][/SUP] In keeping with these studies, the European Male Aging Study (EMAS), a large cross-sectional study involving 3 369 participants, highlighted that many predisposing lifestyle and health factors contributing to the age-related decline in testosterone levels are modifiable and preventable. [SUP][26, 37][/SUP] Overall, these studies do not support the concept of a widespread, inevitable syndrome of age-related sex hormone deficiency in men. The EMAS found a prevalence of late-onset hypogonadism (LOH) of only 2·1% in the general male population and 5·1% in men aged 70–79 years, [SUP][26][/SUP] contrasting with a prevalence for LOH of up to 12% quoted by some pharma promotional material. [SUP][32][/SUP]
Overall, testosterone levels do not correlate well with the symptoms of hypogonadism in older men with LOH, [SUP][26][/SUP] and there is a lack of compelling evidence from large-scale RCTs on the benefits of TRT in ageing men. [SUP][15–18][/SUP] While reduced muscle mass and bone mineral density are clearly established features of organic/syndromic hypogonadism, there is inadequate evidence for a causal relationship between the similar physiological changes observed in older men with FHH. Studies of TRT performed in frail older men have given conflicting results in relation to muscle strength, mood, sexual function and quality of life. [SUP][15–18][/SUP] Moreover, restoring testosterone level in older men to the 'normal range' risks rendering them 'supraphysiologically' replaced. Older men exhibit slower clearance of steroid hormones and are more susceptible to androgen-induced polycythaemia and consequent thrombosis risk, as shown by a meta-analysis including 19 randomized, placebo-controlled studies. [SUP][38][/SUP] Finally, the potential risks of converting a clinically inapparent focus of prostate carcinoma into a more aggressive lesion remain unquantified.
There are good data to suggest maintaining thyroid hormone levels at a lower 'normal range' is beneficial for older people, [SUP][39][/SUP] and the same principle might conceivably also hold true for testosterone. For instance, it was shown that aggressive testosterone replacement in frail men aged 65 years and above was associated with a significantly increased rate of cardiovascular events. [SUP][25][/SUP]
Therefore, GPs should recognize that many of the proposed beneficial effects ascribed to TRT in older men with putative LOH remain unproven and that the potential long-term risks remain uncharacterized at present. Additionally, there is quantifiable health burden and cost relating to testosterone administration and monitoring such as digital rectal examinations, prostate-specific antigen and haematocrit measurement.
The various guidelines and consensus statements currently available [SUP][40–43][/SUP] are mostly based on expert committee reports, opinions or small, nonrandomized controlled studies, representing the very lowest category of the evidence-grading scale. They tend not to distinguish very clearly between organic/syndromic hypogonadism (for which there is evidence of benefit from TRT) and age-/comorbidity-related hypotestosteronaemia (FHH, for which there is generally no evidence). Proposed treatment thresholds are therefore arbitrary and necessarily vary between the different guidelines. For instance, the Endocrine Society Clinical Practice Guideline is unprescriptive in relation to testosterone treatment for older men with low serum testosterone concentration and to the exact treatment threshold. [SUP][40][/SUP] However, it does caution against a general policy of offering testosterone therapy to all older men with low testosterone levels, with the decision to treat needing to be made by clinicians on an individual patient basis.
Although there is no UK national guideline for testosterone prescribing in men, the Society for Endocrinology has recently issued a valuable position statement on male hypogonadism and ageing. [SUP][43][/SUP] This usefully states that, although TRT has been used effectively for many years in younger patients with classical hypogonadism without major adverse events, the same experience should not be extrapolated to the area of LOH, which is still lacking in longer-term studies of sufficient power to establish clinical outcomes.
Conclusion
These data have provided a unique perspective into current testosterone prescribing trends in UK. It is likely that an increasing number of men in the UK might be receiving testosterone therapy without a clearly established indication, or even without a robust clinical diagnosis of hypogonadism. Importantly, there is a general lack of consensus on the threshold to treat in men presenting with nonspecific symptoms and borderline-low serum testosterone level. The paucity of data available to define the 'age-specific' range for the older men and the threshold to treat in 'LOH' underscore the need for large-scale RCTs (or at least, long-term patient registries) to monitor clinical outcomes. Given the shortcomings of currently available international guidelines, consideration should be given to developing a UK-based national guideline that clearly distinguishes between organic and functional causes of hypotestosteronaemia, rather than attempting to set semi-arbitrary serum testosterone thresholds, potentially building on the Society for Endocrinology's existing excellent position statement.
Earn H. Gan, Stewart Pattman, Simon H. S. Pearce, Richard Quinton
Clin Endocrinol. 2013;79(4):564-570.
Abstract
Context Testosterone replacement therapy is the standard treatment for male hypogonadism. There has lately been increased marketing in the medical media promoting testosterone replacement for men with erectile dysfunction or for older men with low serum testosterone, despite the lack of long-term safety and efficacy data. Therefore, we aimed to examine trends in testosterone prescribing in UK primary care over the last 10 years.
Methods Data about the use of testosterone preparations from the Departments of Health Prescription Cost Analysis for community pharmacies 2001–2010, for England, Scotland and Wales, were collated. Community requests for serum total testosterone assay in men to the Biochemistry Department at the Newcastle upon Tyne Hospitals Trust were also examined over the same time period.
Results The number of prescriptions for testosterone preparations increased by nearly 90% from 157 602 to 298 134 dispensed items annually, over a 10-year period. However, due to a particularly significant (fivefold) increase in prescribing of (more expensive) transdermal preparations, the cost to the NHS showed a 267% escalation, from £3·2 to £11·7 million, annually over the same period.
Local requests from primary care in the Newcastle and North Tyneside area for serum testosterone measurement in men also increased, from 347 requests in 2000 to 823 requests in 2010, a 137% increase. However, the number of men with likely unequivocal hypogonadism (testosterone less than 6·0 nm) remained constant at 5·2% in 2000 and 6·3% in 2010.
Conclusion Many men in the UK might be receiving testosterone replacement therapy with neither clearly established indications nor robustly diagnosed hypogonadism. A national registry for men treated with testosterone and further evidence to improve current guidance (national and/or international) on the indications for testosterone replacement would be beneficial.
Background
Human efforts to restore virility and to reverse the effects of ageing in men have been documented since ancient times. As early as 1849, Adolph Berthold postulated a connection between testicular secretions and male behavioural and sexual characteristics from his testicular transplantation experiments. [SUP][1][/SUP] When testosterone was chemically synthesized in 1935, it marked the beginning of a new era in men's health. While the only validated indication for testosterone treatment is male hypogonadism with pathological androgen deficiency, there has been an increasing focus on its role as a 'function-promoting' therapy in older or obese men who wish to restore virility or male character, despite the lack of sound clinical evidence to support this practice.
Male hypogonadism is a clinical syndrome resulting from failure of the testis to produce physiological levels of testosterone and/or maintain normal spermatogenesis, due to disruption of one or more levels of the hypothalamic–pituitary–testis (HPT) axis. Features of hypogonadism include sexual dysfunction, loss of muscle bulk, central obesity, fatigue and mood/sleep disturbances. Long-term complications such as osteoporosis, low-impact fracture and chronic anaemia occur in untreated cases. [SUP][2][/SUP] Although these features are all associated with low serum testosterone concentration, not all men with a low serum testosterone have organic hypogonadism, and there is a large symptom overlap with obesity and nonendocrine illness. For instance, obesity is associated with progressively lower total and free serum testosterone independent of simultaneous reductions in sex hormone binding globulin (SHBG). [SUP][3][/SUP] Lower luteinizing hormone (LH) levels are also seen in obese men, suggesting a potential failure in the hypothalamus–pituitary level. [SUP][3][/SUP] Additionally, the HPT axis exhibits diurnal variation in activity and tends to physiologically shutdown with any form of acute stress, including strenuous physical exertion and sleep deprivation, [SUP][4][/SUP] or chronic disease, such as depression, malnutrition and obesity. There is even an acute suppressive effect of oral glucose loading. [SUP][5][/SUP] Importantly, the HPT axis invariably recovers once these modifiable factors are corrected, [SUP][6][/SUP] hence our preferred term 'functional' hypogonadotropic hypotestosteronaemia (FHH) of nongonadal illness.
Chronic use of prescription and/or nonprescription drugs can also induce hypogonadism, mostly through HPT axis suppression, including opioids, [SUP][7][/SUP] tricyclic antidepressants and selective serotonin reuptake inhibitors, [SUP][8][/SUP] antidopaminergic drugs with hyperprolactinaemic effect such as phenothiazines [SUP][8][/SUP] and risperidone, [SUP][9][/SUP] and antiandrogens such as spironolactone, finasteride [SUP][10][/SUP] and synthetic androgens/anabolic steroids. [SUP][11][/SUP] Combined primary and secondary hypogonadism is seen in some men with a history of excessive alcohol or cannabinoid consumption. [SUP][12, 13][/SUP] A recent double-blind randomized placebo-controlled study (RCT) demonstrated that pioglitazone significantly reduced total and (to an even greater degree) free testosterone levels in eugonadal men with type 2 diabetes. [SUP][14][/SUP]
Hence, testosterone replacement therapy (TRT) may not be appropriate for men with mild, functional and/or transient nonsyndromic hypotestosteronaemia, termed by us FHH, particularly older men with multiple comorbidities for whom treating the primary problem would be more appropriate. Nevertheless, despite the lack of long-term safety data and the inconsistent findings of case–control studies and small RCTs involving older men, [SUP][15–18][/SUP] there have lately been (to our eyes) aggressive marketing campaigns promoting TRT, targeting both the general public and primary care physicians who have sought to equate erectile dysfunction or 'hypogonadism' with 'low serum testosterone'. Therefore, we have set out to uncover the trend and the changes in testosterone prescribing in the primary care setting over the past 10 years.
Methods
Trends in Testosterone Hormone Prescribing: England, Scotland and Wales, 2000–2010
Data on the prescribing trends of testosterone therapy in England, Scotland and Wales were collated by analysing prescription cost analysis (PCA) statistics, data published annually by the Department of Health (DoH). Available data comprised the number of dispensed items from community pharmacists in these regions and the net ingredient costs. These data are based on information obtained from prescriptions sent to the NHS Prescription Pricing Division for payment and include prescriptions dispensed in the community by general practitioners, pharmacists, appliance contractors, prescriptions from hospital doctors dispensed in the community and items personally administered by doctors. We analysed PCA data for England for the calendar years 2000–2010, inclusive and for 2001–2010, inclusive for Scotland and Wales. The trend in testosterone usage on a year-by-year basis can be ascertained by analysing the PCA data. For testosterone products () introduced to the market after the year 2001 ( e.g. testosterone gel preparations and Nebido injection), we included the first full year of availability as the baseline in our analysis (e.g. we used cumulative data from years 2003–2004 as the baseline for Testogel, the lead testosterone in the UK, which was introduced in 2003).
Table 1. Testosterone products and cost per month according to product types
| Generic name | Brand name | Formulation | NIC per quantity (£)* | Maintenance dosage as per BNF | Cost per month-30 days (£) |
|---|---|---|---|---|---|
| Testosterone gel | Testim gel | 50 mg/5 g tube | 1·07 | 50 mg/day | 32·0 |
| Testogel | 50 mg/5 g sachet | 1·04 | 50 mg/day | 31·2 | |
| Tostran 2% gel (60 g gel/dispenser) | 10 mg/0·5 g gel/metered application | £26·67/60 g multidose dispenser | 60 mg–80 mg/day | 40–53·3 (1·5–2 dispenser/month) | |
| Testosterone | Testosterone implant | 100 mg | 7·40 | 600 mg every 4–5months | 8·9–11·1 |
| Testosterone implant | 200 mg | 13·79 | 600 mg every 4–5 months | 8·3–10·3 | |
| Testosterone enanthate | Testosterone enanthate injection | 250 mg/ml/amp | 13·33 | Every 3–6 weeks | 8·9–17·8 |
| Testosterone esters | Sustanon injection | 250 mg/ml/amp | 2·45 | Every 3 weeks | 3·3–4·9 |
| Testosterone propionate | Virormone injection | 100 mg/2 ml/amp | 4·50 | 100 mg–150 mg weekly | 18–27 |
| Testosterone undecanoate | Nebido injection | 1 g/4 ml/amp | 80 | Every 10–14 weeks | 22·9–32 |
| Restandol testocaps | 40 mg | 0·29 | 40–120 mg/day | 8·7–26·1 |
<TBODY>
</TBODY>
Local Community Requests for Serum Total Testosterone Assay in Men, 2000–2010
The number of primary care requests from 2000 to 2010, inclusive, for total testosterone assay in men to the Biochemistry Department at the Newcastle upon Tyne Hospitals Trust (NuTH) was examined by interrogating the laboratory database using the Cognos Impromptu software (version 7.1, IBM Software, Armonk, USA). During this period, total testosterone was quantified using an automated competitive immunoassay. NuTH comprises hospitals delivering secondary care to the Tyneside area, serving a primary care population of 310 000 between 52 GP practices, and tertiary care across North East England and Cumbria. Our survey aimed to explore the trend in the number of serum testosterone assays for men requested by Tyneside GPs and the pattern of results generated from these requests.
Results
Analysis of Testosterone Hormone Usage in England (2000–2010), Wales and Scotland (2001–2010)
The number of prescription items dispensed for all forms of testosterone preparations in England, Wales and Scotland has increased by nearly 90% from 157 602 to 298 134 prescriptions, between 2001 and 2010.(Fig. 1 panels a & b). The cost of this medication to the NHS showed a remarkable 267% escalation, from £3·2 to £11·7 million over the same period. (Fig. 1, panels c & d). The trend in testosterone prescribing for all three regions is highly congruent, seeing a marked increase in the amount of prescriptions for both transdermal preparations and testosterone undecanoate injection. The rising pattern was most significant for transdermal preparations with a fivefold increase, from 28 247 to 151 676 dispensed items, and a 360% escalation in annual expenditure (£1·46 million–£6·74 million, 2001–2010) (Fig. 2).

Figure 1.
Quantity and cost of testosterone prescribing in England, Wales and Scotland. Panel a & b: The number of prescription items for all testosterone replacement therapies, from 2001 to 2010. Panel c & d: Net ingredient cost for testosterone replacement, from 2001 to 2010.

Figure 2.
The number of prescribed items for testosterone replacement according to subtypes in England, Wales and Scotland, 2001–2010.
When transdermal preparation prescriptions were analysed according to their subtypes, a significant sharp rise was observed in testosterone gel since their introduction in 2003, with a 3-fold increase, from 32 300 to 105 300 dispensed items, and 238% escalation in annual expenditure (£1·3 million–£4·54 million; year 2003/2004–2010), as per PCA data in England. On the other hand, the use of some other testosterone preparations, including transdermal patches, mucoadhesive buccal tablets and pellets for subcutaneous implant declined steadily after the introduction of transdermal testosterone gel products.
Of the three currently available testosterone gel products (Testim® [Ferring Pharmaceuticals, West Drayton, UK], Tostran-2%® [ProStrakan, Galashiels, UK] and Testogel® [Bayer Schering, Newbury, Berkshire, UK]), the latter is the most commonly prescribed product, representing 80% of testosterone gel prescribed (2010), constituting 64% of the total expenditure for all types of testosterone in 2010. The overall usage of gels has increased from 32 300–84 900 dispensed items (163%) since the 12-month period following its introduction of the lead product (Testogel®) in 2003.
The PCA data in England also revealed that the use of testosterone undecanoate has been on the rise over the past 10 years, from 31 336 to 65 998 items (111%; 2000–2010). This is solely attributed to the introduction of long-acting testosterone depot injection, Nebido® (Bayer Schering), for which there has been a major rise in prescriptions since its introduction in 2005, with a 365% escalation in dispense items (8 200–38 100; 2005/2006–2010) and a 4·5-fold increase in overall expenditure over the same period (£735 500–£3·29 million). The oral preparation of testosterone undecanoate (Restandol capsule®, Merck Sharp & Dohme Limited, Hoddesdon, Herts, UK) showed a modest fall in the last 10 years (27 400–22 900 items; 2001–2010).
The use of short-acting testosterone injection (testosterone enanthate, testosterone ester and testosterone propionate) has remained relatively stable over the past decade. Sustanon® (Merck Sharp & Dohme Limited) 100 mg (testosterone ester) and Andropatch® (GlaxoSmithKline, Brentford, Middlesex, UK) (transdermal testosterone) have been withdrawn from the UK market since 2010.
Analysis of Local Community Requests for Serum Total Testosterone Assay in Men, 2000–2010
Local requests for serum testosterone measurement in men from primary care in the NuTH Biochemistry Department have increased from 347 requests in 2000 to 823 requests in 2010, with a 137% rise (Fig. 3, panel a). However, the number of men with a high likelihood of hypogonadism (testosterone less than 6·0 nm) remained relatively constant at 5·2% in 2000 and 6·3% in 2010. On the other hand, the number of men with total testosterone in the 6–8·9 nm range, where there is greater uncertainty in the diagnosis of hypogonadism, increased by over 2-fold, from 7·51% to 16·99%. (Fig. 3, panel b).

Figure 3.
Local requests for testosterone level in males at Royal Victoria Infirmary. Panel a. The number of GP requests for testosterone level. Panel b. The trend of serum testosterone level from the local requests.
Discussion
The PCA data from England, Wales and Scotland have clearly shown a progressive rise in the prescribing of testosterone from 2001–2010. This is in keeping with the data available globally, including Switzerland, [SUP][19][/SUP] United States [SUP][20][/SUP] and Australia, [SUP][21][/SUP] suggesting a potential 'pandemic' of testosterone prescribing. There are a number of potential explanations for this trend. Firstly, conditions associated with hypogonadism might be on the rise. For example, the birth prevalence per 100 000 men with Klinefelter syndrome (KS), the most common pathological cause of androgen deficiency, is reported as having risen from 109 in the 1960s–1970s to 223 during the period of 1986–2006. [SUP][22, 23][/SUP] There has been a definite increase in the number of childhood and adult cancer survivors, comprising men who have received radiotherapy to the pituitary or testis or have been orchidectomized for seminoma or teratoma of the testis. Finally, opioids such as tramadol and slow-released morphine sulphate tablets are now more widely used for the treatment of chronic noncancer pain. However, the nearly twofold increase in testosterone prescribing is highly unlikely to be solely attributable to an increased prevalence of male hypogonadism, especially as most men with KS, the commonest cause of pathological male hypogonadism, only become hypogonadal later in life. [SUP][24][/SUP]
Another possible explanation for the observed prescribing trend is increased testosterone testing in primary care, which could potentially result in either improved diagnosis of male hypogonadism and/or an unwarranted increase in TRT in men with borderline serum testosterone concentrations. It is entirely appropriate to treat symptomatic men with borderline-low testosterone under defined circumstances, such as gonadotrophin deficiency resulting from pituitary tumours or prescribed opiate analgesia, or elevated gonadotrophins diagnostic of primary testicular dysfunction ( e.g. KS). However, with these important exceptions, there is insufficient evidence for initiating TRT in most men with a borderline-low serum testosterone due to FHH. There are likewise no data to support testosterone therapy in the context of age-related frailty. [SUP][3, 25, 26][/SUP] We simply do not know whether borderline biochemical hypotestosteronaemia related to FHH is maladaptive, neutral or conceivably even adaptive.
Our local survey has demonstrated that the absolute number of men with likely unequivocal hypogonadism (testosterone <6·0 nm) has remained relatively constant despite a surge in testosterone testing in primary care. The rising number of requests has identified significantly more men with testosterone level in the 6–9 nm range, although 9 nm is the lower limit of the adult male normal range in our assay. Admittedly, this local pattern of requests and results might not reflect the overall situation in the UK, but there are currently no other comparable published data available.
Finally, a plausible explanation for the observed trend in testosterone prescribing in the UK is that an increasing number of eugonadal men might be receiving unnecessary testosterone therapy, particularly older men with 'nongonadal' illness. Although the newer testosterone preparations have undoubtedly improved the quality of life for many men with organic hypogonadism, due to ease of use and better pharmacokinetic profile, pharma has promoted TRT to a broader population of older men with sexual dysfunction. [SUP][27, 28][/SUP] Over the past 3 years, the advertising tracker Kantar Media has reported an increase of more than 170% in spending on advertising by pharmaceutical companies such as Abbott and Eli Lilly in the USA to promote TRT. [SUP][29][/SUP]
Although direct-to-consumer pharmaceutical marketing is not permitted in the UK, the public has virtually unfettered access to pharma websites as key sources of information relating to ageing, erectile dysfunction and the 'andropause'. [SUP][30–33][/SUP] A general theme from pharma-hosted online sources is that testosterone deficiency is common and particularly so with advancing age. Hence, it should be no surprise if men with nonspecific, physiological, age- or illness-related symptoms should increasingly consider these as possible manifestations of testosterone deficiency, with a consequent belief that TRT might restore their quality of life and virility. The question is whether this information campaign has over-reached the actual evidence?
It is well established that a significant portion of older men have testosterone levels below the lower limit for healthy, young men, with an average decline in serum testosterone levels of 1–2% per year. [SUP][34][/SUP] Although this has historically been attributed to physiological decline in testosterone production with age, a large 5-year longitudinal study involving 1 382 community-dwelling men reported that the testosterone decline seen in older men was actually largely caused by a combination of an increased burden of nonendocrine disease and modifiable factors, such as obesity and lifestyle, rather than being an inevitable fact of ageing per se. [SUP][35][/SUP] Indeed, the Odense Androgen Study demonstrated similar serum testosterone reference intervals for both young and elderly healthy men. [SUP][36][/SUP] In keeping with these studies, the European Male Aging Study (EMAS), a large cross-sectional study involving 3 369 participants, highlighted that many predisposing lifestyle and health factors contributing to the age-related decline in testosterone levels are modifiable and preventable. [SUP][26, 37][/SUP] Overall, these studies do not support the concept of a widespread, inevitable syndrome of age-related sex hormone deficiency in men. The EMAS found a prevalence of late-onset hypogonadism (LOH) of only 2·1% in the general male population and 5·1% in men aged 70–79 years, [SUP][26][/SUP] contrasting with a prevalence for LOH of up to 12% quoted by some pharma promotional material. [SUP][32][/SUP]
Overall, testosterone levels do not correlate well with the symptoms of hypogonadism in older men with LOH, [SUP][26][/SUP] and there is a lack of compelling evidence from large-scale RCTs on the benefits of TRT in ageing men. [SUP][15–18][/SUP] While reduced muscle mass and bone mineral density are clearly established features of organic/syndromic hypogonadism, there is inadequate evidence for a causal relationship between the similar physiological changes observed in older men with FHH. Studies of TRT performed in frail older men have given conflicting results in relation to muscle strength, mood, sexual function and quality of life. [SUP][15–18][/SUP] Moreover, restoring testosterone level in older men to the 'normal range' risks rendering them 'supraphysiologically' replaced. Older men exhibit slower clearance of steroid hormones and are more susceptible to androgen-induced polycythaemia and consequent thrombosis risk, as shown by a meta-analysis including 19 randomized, placebo-controlled studies. [SUP][38][/SUP] Finally, the potential risks of converting a clinically inapparent focus of prostate carcinoma into a more aggressive lesion remain unquantified.
There are good data to suggest maintaining thyroid hormone levels at a lower 'normal range' is beneficial for older people, [SUP][39][/SUP] and the same principle might conceivably also hold true for testosterone. For instance, it was shown that aggressive testosterone replacement in frail men aged 65 years and above was associated with a significantly increased rate of cardiovascular events. [SUP][25][/SUP]
Therefore, GPs should recognize that many of the proposed beneficial effects ascribed to TRT in older men with putative LOH remain unproven and that the potential long-term risks remain uncharacterized at present. Additionally, there is quantifiable health burden and cost relating to testosterone administration and monitoring such as digital rectal examinations, prostate-specific antigen and haematocrit measurement.
The various guidelines and consensus statements currently available [SUP][40–43][/SUP] are mostly based on expert committee reports, opinions or small, nonrandomized controlled studies, representing the very lowest category of the evidence-grading scale. They tend not to distinguish very clearly between organic/syndromic hypogonadism (for which there is evidence of benefit from TRT) and age-/comorbidity-related hypotestosteronaemia (FHH, for which there is generally no evidence). Proposed treatment thresholds are therefore arbitrary and necessarily vary between the different guidelines. For instance, the Endocrine Society Clinical Practice Guideline is unprescriptive in relation to testosterone treatment for older men with low serum testosterone concentration and to the exact treatment threshold. [SUP][40][/SUP] However, it does caution against a general policy of offering testosterone therapy to all older men with low testosterone levels, with the decision to treat needing to be made by clinicians on an individual patient basis.
Although there is no UK national guideline for testosterone prescribing in men, the Society for Endocrinology has recently issued a valuable position statement on male hypogonadism and ageing. [SUP][43][/SUP] This usefully states that, although TRT has been used effectively for many years in younger patients with classical hypogonadism without major adverse events, the same experience should not be extrapolated to the area of LOH, which is still lacking in longer-term studies of sufficient power to establish clinical outcomes.
Conclusion
These data have provided a unique perspective into current testosterone prescribing trends in UK. It is likely that an increasing number of men in the UK might be receiving testosterone therapy without a clearly established indication, or even without a robust clinical diagnosis of hypogonadism. Importantly, there is a general lack of consensus on the threshold to treat in men presenting with nonspecific symptoms and borderline-low serum testosterone level. The paucity of data available to define the 'age-specific' range for the older men and the threshold to treat in 'LOH' underscore the need for large-scale RCTs (or at least, long-term patient registries) to monitor clinical outcomes. Given the shortcomings of currently available international guidelines, consideration should be given to developing a UK-based national guideline that clearly distinguishes between organic and functional causes of hypotestosteronaemia, rather than attempting to set semi-arbitrary serum testosterone thresholds, potentially building on the Society for Endocrinology's existing excellent position statement.

