jimbosmith316
MuscleChemistry
TUDCA is a water soluble bile acid. It shows great potency in treating cholestasis (bile acid backup in the liver) as the water soluble bile acids counteract the toxicity of regular bile acids. Can also protect and rehabilitate the liver, and general protects cells; very promising molecule.This page features 67 unique references to scientific papers. History Discussion
Summary
All Essential Benefits/Effects/Facts & Information
Tauroursodeoxycholic acid, more commonly referred to as TUDCA, is a bile salt that is found natrually occurring in the body. When regular bile salts reach the intestines, they can be metabolized by bacteria into UDCA and then later bound to a Taurine molecule to become TUDCA.
TUDCA is a water-soluble bile salt, which is in contrast to regular bile salts possessing both water soluble and fat soluble ends and conferring a detergent effect. This is good for the bile salt's biological purpose (emulsifying fats in the intestines to help with absorption) but when bile acids back up in the liver, a clinical state called cholestasis which occurs when the liver is unhealthy, these bile salts can be damaging to cells by destroying the membranes and signalling for cell death. TUDCA and other water soluble bile salts like UDCA compete with this toxicity and thus indirectly protect cells from death.Additionally, it seems that TUDCA is able to reduce stress to any cell's Endoplasmic Reticulum; an organelle in cells that serves as a highway from the nucleus out into the cytoplasm, and aids in folding proteins. Through reducing ER stress, TUDCA has been implicated in a wide range of beneficial metabolic effects such as reducing insulin resistance and diabetes, and being a neurological protection agent. However, usages of TUDCA beyond the liver are preliminary whereas usage of TUDCA for helping an already harmed liver is quite reliable as TUDCA is used in clinical settings (hospitals) for treating cholestasis.
Things To Know
Also Known As
TUDCA
Do Not Confuse With Taurine (a moiety on the UDCA part)
Things to Note
TUDCA is water-soluble
Caution Notice
If using TUDCA for treating an alcohol-abused liver, be aware of the temporal relationship needed. Co-incubation (same time) or rehabilitative (after the matter) usage of TUDCA may be protective of the liver while pre-loading TUDCA before drinking may be harmful to the liver.Examine.com Medical
Disclaimer
How to Take Recommended dosage, active amounts, other details 10-13mg daily has once been shown to improve liver regenesis rates in a clinically ill population, and may be the lowest estimate of an active oral dose. When looking at improving bile salt composition, a dose around 15-20mg/kg bodyweight TUDCA seems best according to one study.
Benefits have been seen at 1,750mg daily for muscle and liver insulin sensitivity, which is the highest dose used for treatment of fatty liver disease.<header style="box-sizing: border-box; padding-bottom: 55px; color: rgb(71, 71, 71); font-family: "Open Sans", sans-serif; font-size: 14px;">
Human Effect Matrix
The Human Effect Matrix looks at human studies (it excludes animal and in vitro studies) to tell you what effects tauroursodeoxycholic acid has on your body, and how strong these effects are.</header>
<tbody>
</tbody>
<tbody>
</tbody><article id="full_summary" class="supplement-class-0" style="box-sizing: border-box; padding-top: 100px; line-height: 1.52; color: rgb(71, 71, 71); font-family: "Open Sans", sans-serif; font-size: 14px;"><header style="box-sizing: border-box; padding-bottom: 55px;">
</header></article>
- - - Updated - - -
<article id="full_summary" class="supplement-class-0" style="box-sizing: border-box; padding-top: 100px; line-height: 1.52; color: rgb(71, 71, 71); font-family: "Open Sans", sans-serif; font-size: 14px;"><header style="box-sizing: border-box; padding-bottom: 55px;">Sources and Structure
</header>1.1. Sources
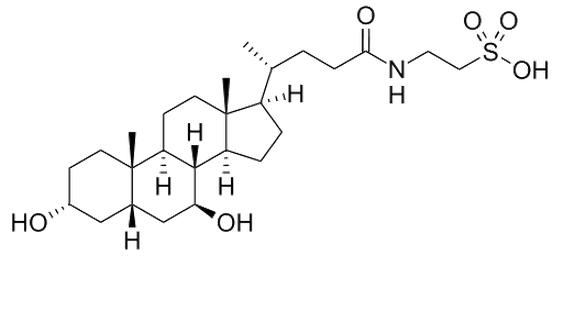
Tauroursodeoxycholic Acid (Tauro- Urso- Deoxy- Cholic), commonly referred to as TUDCA, is a derivative of a secondary bile salt secreted from the liver. When bile salts created by the liver reach the intestines, they can be converted into UDCA (ursodeoxycholic acid) by intestinal bacteria[1] and TUDCA is created when a Taurine molecule is added to the structure.[2] Both of these related molecules are known as hydrophilic bile acids.[2]It is a common constituent of bile acids, and is in high amounts in the bile acids of bears where is has traditionally been extracted.1.2. Structure
 <header style="box-sizing: border-box; padding-bottom: 55px;">
<header style="box-sizing: border-box; padding-bottom: 55px;">
</header>2.1. Absorption and bioavailability
After oral administration, TUDCA appears to be more effective in raising bile concentrations of UDCA (and downstream effects, such as reducing liver enzyme levels) than UDCA itself; this is most likely due to theTaurine group enhancing bioavailability.[3][4] The process of conjugating UDCA with taurine is a rate-limiting step, and this rate-limit is avoided with supplementation of TUDCA.[5]2.2. Distribution
Orally administered TUDCA, at 750mg daily, was able to significantly change the UDCA content of serum, fecal, and urinary bile measurements after 2 months of supplementation; suggesting systemic distribution.[3] Only a small amount of UDCA in serum is unconjugated, as most is bound to taurine or glycine; oral administration of 1500mg for 6 months has an unconjugated UDCA contant of 0.6+/-0.3% in serum.[4]Orally administered TUDCA is able to reach neuronal tissue in rats.[6]<header style="box-sizing: border-box; padding-bottom: 55px;">
</header>The endoplasmic reticulum (ER) is an organelle in cells that extends from the nucleus and forms an interconnected network of pathways in the cell. The ER has a role in folding protein structures, and oxidative stress can cause the ER to misfold or unfold proteins in a response called the 'Unfolding Protein Response' (UPR); which is an adaptive self-protective response in cells, either reversing the stressor or (if it fails to do so) signalling cell death.[7] TUDCA is seen as an ER stress response attenuator, and is able to block cell death induced by the adaptive UPR and preserve cell function.[2][8][9]3.1. ER stress and Ischemia/Reperfusion
Via reducing the unfolding protein response, TUDCA has been shown to reduce damage to organs from acute oxygen deprivation such as acute kidney trauma,[10] surgery on the liver,[9][11][12] and stroke.[13] It has also been shown to inhibit neovascularization in diabetic rats, which is induced by similar mechanisms above.[14]<header style="box-sizing: border-box; padding-bottom: 55px;">
</header>Endoplasmic Reticulum (ER) stress appears to be a significant regulator in liver cells (hepatocytes) of inflammation and injury[12] and in part insulin signalling.[15] Tauroursodeoxycholic Acid (TUDCA), as a suppressor of protein unfolding and ER stress, may confer a therapeutic and rehabilitative role.4.1. Cell death and count
TUDCA has been shown to improve liver healing rates in both steatotic and non-steatotic livers via ER stress reduction after ischemia/reperfusion.[9] TUDCA, in this study, resulted in less cell death via alleviating a mechanism known to accelerate cell apoptosis and also suppressed the actions of IRE-1 andPKR-like ER kinase, two pathways known to induce protein unfolding.[9] This cell protective effect has also been seen in liver cells undergoing cold storage[16] as well as the epithelial cells of bile ducts, which are damaged during chronic hepatitis.[17] These latter benefits are mediated via PKCa and intracellular Ca2+[17] and may be dependent on basic levels of dietary methionine and Choline.[18]After administration in men with chronic liver disease, 10-13mg TUDCA daily for 3 months appears to enhance the rate of hepatocyte proliferation as well.[19]
Cholestasis is a condition in which bile flow from the liver is distrupted in some manner. UDCA and TUDCA are currently one of the first lines of treatment for a variety of cholestatic syndromes.[2]Accumulation of bile acids in the liver during periods of impeded intestinal secretion (cholestasis) can cause cell death in hepatocytes secondary to the bile acid's detergent-like effects,[20][21][22] and UDCA/TUDCA confer protective effects in this scenario. The detergent-like effects are related to bile acid's hydrophobicity and lipid solubilizing actions, yet UDCA/TUDCA are highly hydrophilic and unlikely to possess the same toxicity.[23][24]The mechanism of protection may be through competitive antagonism of harmful bile salts actions on hepatocytes.[25] This, in conjunction with TUDCA supplementation increasing the relative amount of hydrophilic bile salts (UDCA, TUDCA) relative to the harmful salts exerts an overall protective effect. The increase in UDCA seen with supplementation is a diminishing response dose-dependent relationship with 500mg, 1000mg, and 1500mg increasing the bile content of TUDCA from 2.9% (in persons with Primary Biliary Cirrhosis) to 34.4+/-4.5%, 32.8+/-2.8%, and 41.6+/-3%, respectively.[4] The most effective dose for this beneficial partitioning of bile salts, based on plotted curves from serum and bile analysis, may be 15-20mg/kg bodyweight TUDCA.[4]
Gallstones (made of cholesterol) may be treated in a litholytic manner with bile acids,[26] dissolving them to a size in which they may be passed. The process of dissolving gallstones made of cholesterol is sometimes also referred to as cholelitholytic[27] (with litholytic being a more general term, and used with more frequency for kidney stones). Cholesterol gallstones are approximately 75% of all hepatic gallstones (at least in Western countries[28][29]) and are thought to be the only formation of gallstone able to be dissolved with bile acids.[30]Gallstones may become opaque, which is thought to indicate their inability to dissolve (and thus, a failure of litholysis treatment);[31] the opacity appears to be related to calcium deposition in the cholesterol gallstone, and appears to make gallstones resistant to bile acid induced cholelitholysis.[32][30] The opaque gallstones are either black (around 20% in total and consisting of calcium phosphate and/or carbonate with insoluble bilirubin pigment and cholesterol) or brown (around 5% overall containing calcium bilirubinate, calcium palmitate, stearate and cholesterol).[33][30]
</header>5.1. Huntington's Disease
Oral administration of TUDCA in rats was shown to be protective against a toxin which induces Huntington's Disease,[6][38] and was later shown in rats to be neuroprotective in a model of Huntington's Disease without said toxin; reducing neuronal death and improving symptoms.[39]These protective effects may also extend to dopaminergic neurons and Parkinson's Disease,[40][41] but no practical oral ingestion studies exist.5.2. Alzheimer's
In vitro studies suggest that TUDCA incubation alongside beta-amyloid pigment, an insoluble protein involved in the pathology of Alzheimer's, was able to prevent beta-amyloid induced cell death in rat neuronal and astrocyte cells,[42] neuroblastoma cells,[43] primary corticol neurons,[44] and PC12 cells.[45]Mitochondrial permeability associated with beta-amyloid induced toxicity is decreased with TUDCA[42] and inhibits adverse changes in the mitochondria as a result of beta-amyloid toxicity.[46]However, TUDCA has not been implicated in actually reducing beta-amyloid levels. This suggests protective effects on cells independent of beta-amyloid presence.[2]5.3. Stroke
TUDCA has shown neuroprotective actions against stroke and acute neurological injury[47][13]
</header>6.1. Mechanisms
TUDCA has been demonstrated to restore glucose homeostasis in a cell culture as well as diabetic mice and increase insulin sensitivity in liver, muscle, and adipose tissue.[8] ER stress has been linked to both obesity as well as diabetes,[48] possibly through increased ER stress inducing an adaptive unfolded protein response, and inhibiting insulin signalling via activating the JNK pathway via IRE-1.[49][50] Whether ER stress, per se, is a causative step in muscle tissue is not known and may be doubtful.[51]A study in mice at 0.5mg/kg bodyweight TUDCA suggest that thyroid hormone deiodinase enyzmes, specifically D2, play a role. Supplementation of this dose in rats has been shown to normalize glucose tolerance[8] but this effect is not seen in mice without the D2 enzyme or mRNA (Dio2−/− mice).[52]6.2. Interactions with Beta-cells
An in vitro study on the interactions of TUDCA and beta-cells found that TUDCA failed to normalize insulin resistance when glucose was incubated with a cell at 6.5mmol/L but restored function at higher levels of glycermia (13 and 22mmol/L) without affecting insulin content of the cells.[53] Hyperglycemia was able to reduce mRNA production of preproinsulin (insulin precursor), and this reduction was prevented with TUDCA.[53] 13mmol/L correlates to the cellular concentration of hyperglycemia[54] and is physiologically relevant. These effects seem to be secondary to reducing ER stress.[53]6.3. Interventions
TUDCA, at 1,750mg daily in obese persons without NAFLD nor type II diabetes over 4 weeks, was able to reduce insulin resistance in skeletal muscle as assessed by increased phosphorylation of Akt and IRS-1 in response to a standardized test meal.[55] No influence on insulin induced JNK activation was seen in myocytes.
</header>7.1. Thyroid and Metabolic Rate
Circulating Bile Acids (those not found in the liver, but the blood) have been found to be correlated with energy expenditure in humans. With correlation coefficients of 0.648 in healthy persons and 0.833 in those with cirrhosis,[56] although other smaller studies (n=24) find no such correlations.[57]TUDCA has been shown in vitro to increase protein content and mRNA levels for the deiodinase enzyme D2 in cells expressing this protein normally; this protein converts relatively low-activity thyroid hormone T4 to T3, and incubation with TUDCA was able to double protein content of the enzyme and increase T3 levels at concentrations between 100-800uM, reaching three-fold increases at the highest level.[52]Although the delayed response to max potency and prior evidence that deiodinases are regulated post-transcriptionally,[58] the authors noted that the increase in deiodinase mRNA (Dio2) makes it unlikely that the effects seen are secondary to protein stabilization.[52]When fed to mice at 0.5mg/kg bodyweight, TUDAC was unable to induce fat loss over 7 days but induced a change in respiratory quotient by 5% towards using fatty acids as substrate.[52] Serum levels of T3 and T4 were unchanged in these animals, suggesting localized usage of T3 or a mechanism independent of thyroid hormones.[52] Another report noted small but statistically significant decreases in body weight in mice over 15-30 days, but did not specify dose of TUDCA used.[8] It is possible that these effects are through increasing activity of the D2 receptor, which has been demonstrated in animals with 0.5% cholic acid in the diet that induced similar effects such as an increase in metabolic rate alongside a decrease in respiratory quotient (indicative of a greater % of energy from fatty acids).[59]These mechanisms may be relevant to humans due to muscle cells expressing the same D2 receptor, and at least cholic acid has been found to increase human myocyte mRNA of this receptor (TGR5) and similar trends were found with all tested bile acids.[59]7.2. Adipokines
It has been noted that the obesity-related suppression of adiponectin content may be related to ER stress, and one rat study has shown TUDCA in diet-induced obesity was able to increase circulating adiponectin levels via the protein DsbA-L.[60]7.3. Interventions
After ingestion of 1,750mg TUDCA for 4 weeks in obese but otherwise healthy persons, no significant effects were observed on body fat or weight.[55] Insulin sensitivity was improved in skeletal muscle and the liver, but not in the adipose (body fat) tissue of these persons.[55] This differs from previous rat studies[8]and may be due to the dose in the rat studies being higher.
</header>8.1. Alcohol
Alcohol, or drinking ethanol, can be as fun as it is damaging to the liver.TUDCA, and its taurine-free conjugate UDCA, have been shown to attenuate the reduction of bioenergetics in a liver cell after incubation with acetaldehyde (metabolite of alcohol that does the damage) as well as significantly reduce cell death induced by ethanol when TUDCA is at 0.1mM concentration and UDCA at 0.01mM.[61] Concentrations of 0.5mM of both have shown similar mechanistic protection but slightly weaker,[62] but dose-response is not present as higher concentrations (>0.1mM) were reported to induce cell death.[61] TUDCA appeared to be geared towards preserving membrane function while UDCA was more potent at preserving mitochondrial function.[61]Most critically, these benefits were seen with co-incubation or adminstration of them both at the same time.[62][61] When pre-loaded before ethanolic insult, they have been shown to exacerbate damage to liver cells.[61] These effects may be secondary to alterations in the lipid membrane of cells with TUDCA/UDCA exposure.[63]
</header></article>
Summary
All Essential Benefits/Effects/Facts & Information
Tauroursodeoxycholic acid, more commonly referred to as TUDCA, is a bile salt that is found natrually occurring in the body. When regular bile salts reach the intestines, they can be metabolized by bacteria into UDCA and then later bound to a Taurine molecule to become TUDCA.
TUDCA is a water-soluble bile salt, which is in contrast to regular bile salts possessing both water soluble and fat soluble ends and conferring a detergent effect. This is good for the bile salt's biological purpose (emulsifying fats in the intestines to help with absorption) but when bile acids back up in the liver, a clinical state called cholestasis which occurs when the liver is unhealthy, these bile salts can be damaging to cells by destroying the membranes and signalling for cell death. TUDCA and other water soluble bile salts like UDCA compete with this toxicity and thus indirectly protect cells from death.Additionally, it seems that TUDCA is able to reduce stress to any cell's Endoplasmic Reticulum; an organelle in cells that serves as a highway from the nucleus out into the cytoplasm, and aids in folding proteins. Through reducing ER stress, TUDCA has been implicated in a wide range of beneficial metabolic effects such as reducing insulin resistance and diabetes, and being a neurological protection agent. However, usages of TUDCA beyond the liver are preliminary whereas usage of TUDCA for helping an already harmed liver is quite reliable as TUDCA is used in clinical settings (hospitals) for treating cholestasis.
Things To Know
Also Known As
TUDCA
Do Not Confuse With Taurine (a moiety on the UDCA part)
Things to Note
TUDCA is water-soluble
Caution Notice
If using TUDCA for treating an alcohol-abused liver, be aware of the temporal relationship needed. Co-incubation (same time) or rehabilitative (after the matter) usage of TUDCA may be protective of the liver while pre-loading TUDCA before drinking may be harmful to the liver.Examine.com Medical
Disclaimer
How to Take Recommended dosage, active amounts, other details 10-13mg daily has once been shown to improve liver regenesis rates in a clinically ill population, and may be the lowest estimate of an active oral dose. When looking at improving bile salt composition, a dose around 15-20mg/kg bodyweight TUDCA seems best according to one study.
Benefits have been seen at 1,750mg daily for muscle and liver insulin sensitivity, which is the highest dose used for treatment of fatty liver disease.<header style="box-sizing: border-box; padding-bottom: 55px; color: rgb(71, 71, 71); font-family: "Open Sans", sans-serif; font-size: 14px;">
Human Effect Matrix
The Human Effect Matrix looks at human studies (it excludes animal and in vitro studies) to tell you what effects tauroursodeoxycholic acid has on your body, and how strong these effects are.</header>
| GRADE | LEVEL OF EVIDENCE |
|---|---|
|
| Robust research conducted with repeated double-blind clinical trials |
| Multiple studies where at least two are double-blind and placebo controlled | |
| Single double-blind study or multiple cohort studies | |
| Uncontrolled or observational studies only |
<tbody>
</tbody>
| LEVEL OF EVIDENCE? | OUTCOME | MAGNITUDE OF EFFECT? | CONSISTENCY OF RESEARCH RESULTS? | NOTES |
|---|---|---|---|---|
| Liver Enzymes | VERY HIGHSee all 5 studies | The decrease in liver enzymes associated with cholestasis is quite strong, and TUDCA is a reference drug for these effects | ||
| Cholestasis | VERY HIGHSee study | Is either comparable or exceeds the potency of ursodeoxycholic acid, the reference drug for cholestasis | ||
| Insulin Sensitivity | VERY HIGHSee study | Notable due to reaching near 30% improvement in obese persons and only being localized to muscle tissue and the liver, but not adipose tissue | ||
| Blood Glucose | - | VERY HIGHSee study | No significant influence on fasting glucose levels | |
| Fat Mass | - | VERY HIGHSee study | No significant influence on fat mass in obese persons | |
| Insulin | - | VERY HIGHSee study | No significant influence on fasting insulin levels | |
| Weight | - | VERY HIGHSee study | No significant influence on weight in obese persons | |
| HDL-C | VERY HIGHSee study | A decrease in HDL-C has been noted to be secondary to treating cholestasis | ||
| Liver Cell Content | VERY HIGHSee study | An increase in hepatocyte regeneration has been noted with TUDCA in persons with liver disease | ||
| Total Cholesterol | VERY HIGHSee study | A decrease in total cholesterol in serum has been noted secondary to treating cholestasis |
<tbody>
</tbody>
</header></article>
- - - Updated - - -
<article id="full_summary" class="supplement-class-0" style="box-sizing: border-box; padding-top: 100px; line-height: 1.52; color: rgb(71, 71, 71); font-family: "Open Sans", sans-serif; font-size: 14px;"><header style="box-sizing: border-box; padding-bottom: 55px;">Sources and Structure
</header>1.1. Sources
Tauroursodeoxycholic Acid (Tauro- Urso- Deoxy- Cholic), commonly referred to as TUDCA, is a derivative of a secondary bile salt secreted from the liver. When bile salts created by the liver reach the intestines, they can be converted into UDCA (ursodeoxycholic acid) by intestinal bacteria[1] and TUDCA is created when a Taurine molecule is added to the structure.[2] Both of these related molecules are known as hydrophilic bile acids.[2]It is a common constituent of bile acids, and is in high amounts in the bile acids of bears where is has traditionally been extracted.1.2. Structure

2
Pharmacology</header>2.1. Absorption and bioavailability
After oral administration, TUDCA appears to be more effective in raising bile concentrations of UDCA (and downstream effects, such as reducing liver enzyme levels) than UDCA itself; this is most likely due to theTaurine group enhancing bioavailability.[3][4] The process of conjugating UDCA with taurine is a rate-limiting step, and this rate-limit is avoided with supplementation of TUDCA.[5]2.2. Distribution
Orally administered TUDCA, at 750mg daily, was able to significantly change the UDCA content of serum, fecal, and urinary bile measurements after 2 months of supplementation; suggesting systemic distribution.[3] Only a small amount of UDCA in serum is unconjugated, as most is bound to taurine or glycine; oral administration of 1500mg for 6 months has an unconjugated UDCA contant of 0.6+/-0.3% in serum.[4]Orally administered TUDCA is able to reach neuronal tissue in rats.[6]<header style="box-sizing: border-box; padding-bottom: 55px;">
3
Interactions with the Endoplasmic Reticulum</header>The endoplasmic reticulum (ER) is an organelle in cells that extends from the nucleus and forms an interconnected network of pathways in the cell. The ER has a role in folding protein structures, and oxidative stress can cause the ER to misfold or unfold proteins in a response called the 'Unfolding Protein Response' (UPR); which is an adaptive self-protective response in cells, either reversing the stressor or (if it fails to do so) signalling cell death.[7] TUDCA is seen as an ER stress response attenuator, and is able to block cell death induced by the adaptive UPR and preserve cell function.[2][8][9]3.1. ER stress and Ischemia/Reperfusion
Via reducing the unfolding protein response, TUDCA has been shown to reduce damage to organs from acute oxygen deprivation such as acute kidney trauma,[10] surgery on the liver,[9][11][12] and stroke.[13] It has also been shown to inhibit neovascularization in diabetic rats, which is induced by similar mechanisms above.[14]<header style="box-sizing: border-box; padding-bottom: 55px;">
4
Interactions with the Liver</header>Endoplasmic Reticulum (ER) stress appears to be a significant regulator in liver cells (hepatocytes) of inflammation and injury[12] and in part insulin signalling.[15] Tauroursodeoxycholic Acid (TUDCA), as a suppressor of protein unfolding and ER stress, may confer a therapeutic and rehabilitative role.4.1. Cell death and count
TUDCA has been shown to improve liver healing rates in both steatotic and non-steatotic livers via ER stress reduction after ischemia/reperfusion.[9] TUDCA, in this study, resulted in less cell death via alleviating a mechanism known to accelerate cell apoptosis and also suppressed the actions of IRE-1 andPKR-like ER kinase, two pathways known to induce protein unfolding.[9] This cell protective effect has also been seen in liver cells undergoing cold storage[16] as well as the epithelial cells of bile ducts, which are damaged during chronic hepatitis.[17] These latter benefits are mediated via PKCa and intracellular Ca2+[17] and may be dependent on basic levels of dietary methionine and Choline.[18]After administration in men with chronic liver disease, 10-13mg TUDCA daily for 3 months appears to enhance the rate of hepatocyte proliferation as well.[19]
Beyond TUDCA's primary treatment purpose (Cholestasis, next section) TUDCA also appears to have beneficial modulation of cells in the liver, promoting regeneration and reducing cell death. May not be relevant in an already health liver (due to high regeneration rates already), but nice for unhealthy livers
4.2. CholestasisCholestasis is a condition in which bile flow from the liver is distrupted in some manner. UDCA and TUDCA are currently one of the first lines of treatment for a variety of cholestatic syndromes.[2]Accumulation of bile acids in the liver during periods of impeded intestinal secretion (cholestasis) can cause cell death in hepatocytes secondary to the bile acid's detergent-like effects,[20][21][22] and UDCA/TUDCA confer protective effects in this scenario. The detergent-like effects are related to bile acid's hydrophobicity and lipid solubilizing actions, yet UDCA/TUDCA are highly hydrophilic and unlikely to possess the same toxicity.[23][24]The mechanism of protection may be through competitive antagonism of harmful bile salts actions on hepatocytes.[25] This, in conjunction with TUDCA supplementation increasing the relative amount of hydrophilic bile salts (UDCA, TUDCA) relative to the harmful salts exerts an overall protective effect. The increase in UDCA seen with supplementation is a diminishing response dose-dependent relationship with 500mg, 1000mg, and 1500mg increasing the bile content of TUDCA from 2.9% (in persons with Primary Biliary Cirrhosis) to 34.4+/-4.5%, 32.8+/-2.8%, and 41.6+/-3%, respectively.[4] The most effective dose for this beneficial partitioning of bile salts, based on plotted curves from serum and bile analysis, may be 15-20mg/kg bodyweight TUDCA.[4]
For short term treatment of bile acid complications and cholestasis, TUDCA appears to be very potent and reliable. Used in clinical settings for treatment of cholestasis as well.
4.3. GallstonesGallstones (made of cholesterol) may be treated in a litholytic manner with bile acids,[26] dissolving them to a size in which they may be passed. The process of dissolving gallstones made of cholesterol is sometimes also referred to as cholelitholytic[27] (with litholytic being a more general term, and used with more frequency for kidney stones). Cholesterol gallstones are approximately 75% of all hepatic gallstones (at least in Western countries[28][29]) and are thought to be the only formation of gallstone able to be dissolved with bile acids.[30]Gallstones may become opaque, which is thought to indicate their inability to dissolve (and thus, a failure of litholysis treatment);[31] the opacity appears to be related to calcium deposition in the cholesterol gallstone, and appears to make gallstones resistant to bile acid induced cholelitholysis.[32][30] The opaque gallstones are either black (around 20% in total and consisting of calcium phosphate and/or carbonate with insoluble bilirubin pigment and cholesterol) or brown (around 5% overall containing calcium bilirubinate, calcium palmitate, stearate and cholesterol).[33][30]
Gallstones can form in the liver from cholesterol, and administration of bile acids is thought to dissolve the gallstones to a level where they may be passed in the urine (not painless, but does not require surgery); this dissolution appears to not influence all gallstones, with gallstones having a calcium content being resistant to bile acids
Therapy tends to consist of a nightly dose of bile acids (UDCA or TUDCA) paired with a low cholesterol diet to encourage cholesterol efflux.[34][35] It should be noted that therapy with oral bile acids is not first-line, and that usually a minimal amount of persons with gallstones respond to UDCA or TUDCA (10% or less[36]); standard practise suggests that it be limited to persons unfit for surgery with small (5mm or less diameter) uncalcified and cholesterol rich gallstones.[30]In looking at the rate of failure (formation of opacity during treatment of bile acids), there do not appear to be any significant differences between TUDCA, UDCA, or another bile acid known as CDCA.[37]Treatment of gallstones with TUDCA is used in clinical settings, but is not the first-line treatment and requires certain conditions to be met (small uncalcified gallstone of mostly cholesterol) to be effective. When comparing TUDCA against other bile acids, there do not appear to be significant differences in efficacy
<header style="box-sizing: border-box; padding-bottom: 55px;">5
Interactions with Neurology</header>5.1. Huntington's Disease
Oral administration of TUDCA in rats was shown to be protective against a toxin which induces Huntington's Disease,[6][38] and was later shown in rats to be neuroprotective in a model of Huntington's Disease without said toxin; reducing neuronal death and improving symptoms.[39]These protective effects may also extend to dopaminergic neurons and Parkinson's Disease,[40][41] but no practical oral ingestion studies exist.5.2. Alzheimer's
In vitro studies suggest that TUDCA incubation alongside beta-amyloid pigment, an insoluble protein involved in the pathology of Alzheimer's, was able to prevent beta-amyloid induced cell death in rat neuronal and astrocyte cells,[42] neuroblastoma cells,[43] primary corticol neurons,[44] and PC12 cells.[45]Mitochondrial permeability associated with beta-amyloid induced toxicity is decreased with TUDCA[42] and inhibits adverse changes in the mitochondria as a result of beta-amyloid toxicity.[46]However, TUDCA has not been implicated in actually reducing beta-amyloid levels. This suggests protective effects on cells independent of beta-amyloid presence.[2]5.3. Stroke
TUDCA has shown neuroprotective actions against stroke and acute neurological injury[47][13]
In general, TUDCA appears to be quite protective of cells and this applies to the brain as well. Orally ingested TUDCA is able to reach neuronal tissue, but relevance to humans with TUDCA ingestion has not yet been shown
<header style="box-sizing: border-box; padding-bottom: 55px;">6
Interactions with Glucose metabolism</header>6.1. Mechanisms
TUDCA has been demonstrated to restore glucose homeostasis in a cell culture as well as diabetic mice and increase insulin sensitivity in liver, muscle, and adipose tissue.[8] ER stress has been linked to both obesity as well as diabetes,[48] possibly through increased ER stress inducing an adaptive unfolded protein response, and inhibiting insulin signalling via activating the JNK pathway via IRE-1.[49][50] Whether ER stress, per se, is a causative step in muscle tissue is not known and may be doubtful.[51]A study in mice at 0.5mg/kg bodyweight TUDCA suggest that thyroid hormone deiodinase enyzmes, specifically D2, play a role. Supplementation of this dose in rats has been shown to normalize glucose tolerance[8] but this effect is not seen in mice without the D2 enzyme or mRNA (Dio2−/− mice).[52]6.2. Interactions with Beta-cells
An in vitro study on the interactions of TUDCA and beta-cells found that TUDCA failed to normalize insulin resistance when glucose was incubated with a cell at 6.5mmol/L but restored function at higher levels of glycermia (13 and 22mmol/L) without affecting insulin content of the cells.[53] Hyperglycemia was able to reduce mRNA production of preproinsulin (insulin precursor), and this reduction was prevented with TUDCA.[53] 13mmol/L correlates to the cellular concentration of hyperglycemia[54] and is physiologically relevant. These effects seem to be secondary to reducing ER stress.[53]6.3. Interventions
TUDCA, at 1,750mg daily in obese persons without NAFLD nor type II diabetes over 4 weeks, was able to reduce insulin resistance in skeletal muscle as assessed by increased phosphorylation of Akt and IRS-1 in response to a standardized test meal.[55] No influence on insulin induced JNK activation was seen in myocytes.
Preliminary, but TUDCA appears to be able to protect cells from dysfunction associated with hyperglycemia and may reduce the effects of insulin resistance before they happen (beta-cells) and even therapeutically with the potency of some diabetic pharmaceuticals (in regards to the liver and skeletal muscle)
<header style="box-sizing: border-box; padding-bottom: 55px;">7
Interactions with Fat Mass and Obesity</header>7.1. Thyroid and Metabolic Rate
Circulating Bile Acids (those not found in the liver, but the blood) have been found to be correlated with energy expenditure in humans. With correlation coefficients of 0.648 in healthy persons and 0.833 in those with cirrhosis,[56] although other smaller studies (n=24) find no such correlations.[57]TUDCA has been shown in vitro to increase protein content and mRNA levels for the deiodinase enzyme D2 in cells expressing this protein normally; this protein converts relatively low-activity thyroid hormone T4 to T3, and incubation with TUDCA was able to double protein content of the enzyme and increase T3 levels at concentrations between 100-800uM, reaching three-fold increases at the highest level.[52]Although the delayed response to max potency and prior evidence that deiodinases are regulated post-transcriptionally,[58] the authors noted that the increase in deiodinase mRNA (Dio2) makes it unlikely that the effects seen are secondary to protein stabilization.[52]When fed to mice at 0.5mg/kg bodyweight, TUDAC was unable to induce fat loss over 7 days but induced a change in respiratory quotient by 5% towards using fatty acids as substrate.[52] Serum levels of T3 and T4 were unchanged in these animals, suggesting localized usage of T3 or a mechanism independent of thyroid hormones.[52] Another report noted small but statistically significant decreases in body weight in mice over 15-30 days, but did not specify dose of TUDCA used.[8] It is possible that these effects are through increasing activity of the D2 receptor, which has been demonstrated in animals with 0.5% cholic acid in the diet that induced similar effects such as an increase in metabolic rate alongside a decrease in respiratory quotient (indicative of a greater % of energy from fatty acids).[59]These mechanisms may be relevant to humans due to muscle cells expressing the same D2 receptor, and at least cholic acid has been found to increase human myocyte mRNA of this receptor (TGR5) and similar trends were found with all tested bile acids.[59]7.2. Adipokines
It has been noted that the obesity-related suppression of adiponectin content may be related to ER stress, and one rat study has shown TUDCA in diet-induced obesity was able to increase circulating adiponectin levels via the protein DsbA-L.[60]7.3. Interventions
After ingestion of 1,750mg TUDCA for 4 weeks in obese but otherwise healthy persons, no significant effects were observed on body fat or weight.[55] Insulin sensitivity was improved in skeletal muscle and the liver, but not in the adipose (body fat) tissue of these persons.[55] This differs from previous rat studies[8]and may be due to the dose in the rat studies being higher.
Appears to have mechanisms to augment Thryoid Hormone actions and induce fat loss, but the dose may be too high to be practically relevant
<header style="box-sizing: border-box; padding-bottom: 55px;">8
Nutrient-Nutrient Interactions</header>8.1. Alcohol
Alcohol, or drinking ethanol, can be as fun as it is damaging to the liver.TUDCA, and its taurine-free conjugate UDCA, have been shown to attenuate the reduction of bioenergetics in a liver cell after incubation with acetaldehyde (metabolite of alcohol that does the damage) as well as significantly reduce cell death induced by ethanol when TUDCA is at 0.1mM concentration and UDCA at 0.01mM.[61] Concentrations of 0.5mM of both have shown similar mechanistic protection but slightly weaker,[62] but dose-response is not present as higher concentrations (>0.1mM) were reported to induce cell death.[61] TUDCA appeared to be geared towards preserving membrane function while UDCA was more potent at preserving mitochondrial function.[61]Most critically, these benefits were seen with co-incubation or adminstration of them both at the same time.[62][61] When pre-loaded before ethanolic insult, they have been shown to exacerbate damage to liver cells.[61] These effects may be secondary to alterations in the lipid membrane of cells with TUDCA/UDCA exposure.[63]
Might alleviate alcohol's adverse effects on the liver, but appears to be needed to be taken after drinking and may be damaging if taken before
<header style="box-sizing: border-box; padding-bottom: 55px;">9
</header></article>

